Fecal Indicator 7.1 Bacteria
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Microbiology Laboratory Exercises Third Edition 2020
MICROBIOLOGY Laboratory Exercises Third Edition Keddis & Rauschenbach 2020 Photo Credits (in order of contribution): Diane Davis, Ines Rauschenbach & Ramaydalis Keddis Acknowledgements: Many thanks to those in the Department of Biochemistry and Microbiology, Rutgers University, who have through the years inspired our enthusiasm for the science and teaching of microbiology, with special thanks to Diane Davis, Douglas Eveleigh and Max Häggblom. Safety: The experiments included in this manual have been deemed safe by the authors when all necessary safety precautions are met. The authors recommend maintaining biosafety level 2 in the laboratory setting and using risk level 1 organisms for all exercises. License: This work is licensed under a Creative Commons Attribution- NonCommercial-NoDerivatives 4.0 International License Microbiology Laboratory Exercises Third Edition 2020 Ramaydalis Keddis, Ph.D. Ines Rauschenbach, Ph.D. Department of Biochemistry and Microbiology Rutgers, The State University of New Jersey CONTENTS PAGE Introduction Schedule ii Best Laboratory Practices Iii Working in a Microbiology Laboratory iv Exercises Preparation of a Culture Medium 1 Culturing and Handling Microorganisms 3 Isolation of a Pure Culture 5 Counting Bacterial Populations 8 Controlling Microorganisms 10 Disinfectants 10 Antimicrobial Agents: Susceptibility Testing 12 Hand Washing 14 The Lethal Effects of Ultraviolet Light 15 Selection of Fungi from Air 17 Microscopy 21 Morphology and Staining of Bacteria 26 Microbial Metabolism 30 Enzyme Assay 32 Metabolic -
Page 1 of 27 the Diagram Shows Two Thermometers. the Bulb of Each
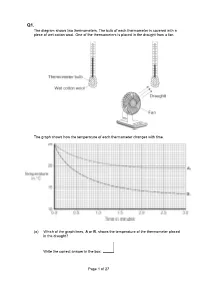
Q1. The diagram shows two thermometers. The bulb of each thermometer is covered with a piece of wet cotton wool. One of the thermometers is placed in the draught from a fan. The graph shows how the temperature of each thermometer changes with time. (a) Which of the graph lines, A or B, shows the temperature of the thermometer placed in the draught? Write the correct answer in the box. Page 1 of 27 Explain, in terms of evaporation, the reason for your answer. ___________________________________________________________________ ___________________________________________________________________ ___________________________________________________________________ ___________________________________________________________________ ___________________________________________________________________ ___________________________________________________________________ (3) (b) A wet towel spread out and hung outside on a day without wind dries faster than an identical wet towel left rolled up in a plastic bag. Explain why. ___________________________________________________________________ ___________________________________________________________________ ___________________________________________________________________ ___________________________________________________________________ (2) (Total 5 marks) Q2. The picture shows a person taking a hot shower. (a) When a person uses the shower the mirror gets misty. Why? ___________________________________________________________________ ___________________________________________________________________ -

Department of Education and Science of Ukraine National University of Food Technologies
DEPARTMENT OF EDUCATION AND SCIENCE OF UKRAINE NATIONAL UNIVERSITY OF FOOD TECHNOLOGIES APPROVED _______________A.I. Ukrainets (signature) «_____» ___________2016 y. V.N. ISCHENKO М.S. SОVКО N.V. ISCHENKO EQUIPMENT AND SAFETY IN CHEMICAL LABORATORY All quotations, digital and actual material, bibliographical information are checked. Writing units respond to the standards Signature (s) of the author (s) _____________________ « _17 » __May__2016 y. Registration number of electronic textbook in EMM 59. 04 – 07. 06. 2016 KIEV NUFT 2016 UDC 542.07 Recommended by the Academic Council of the National University of Food Technologies as learner’s guide for students of higher educational establishments (Minutes № 13 from 31.05. 2016) Reviewers: V.I Maksin – Dr. of Chemistry, Professor of the Department of Bioinorganic Chemistry and Water Quality NUBaN Ukraine O.O. Tkachuck – Ph.D. in Chemistry, the head of the laboratory ofnational anti-doping center of Ukraine O.V. Berezovska – senior lecturer of the Department of Business Foreign Language and International Communication NUFT IschenkoV.N. Equipment and safety in chemical laboratory [Electronic resourse]:learner’s guide /V.N. Ischenko, М.S. Sоvко, N.V. Ischenko. – K.: NUFT, 2016. –27 р. The learner’s guide provides information about modern chemical laboratory equipment. Chemical laboratory ware and equipment necessary for conducting laboratory work are described. The guide provides the rules of behavior and safety in chemical laboratory, rules of work with chemicals Learner’s guide is recommended for students of academic level of bachelor, who study Chemistry in English. Authors: V.N. ISCHENKO, PhD М.S. SОVКО, N.V. ISCHENKO, PhD Introduced in author’s edition © V.N. -

Pouring Plates from Prepared Bottled Media
Pouring Plates from Prepared Bottled Media Primary Hazard Warning Never purchase living specimens without having a disposition strategy in place. When pouring bottles, agar is HOT! Burning can occur. Always handle hot agar bottles with heat-protective gloves. For added protection wear latex or nitrile gloves when working with bacteria, and always wash hands before and after with hot water and soap. Availability Agar is available for purchase year round. Information • Storage: Bottled agar can be stored at room temperature for about six months unless otherwise specified. Never put agar in the freezer. It will cause the agar to breakdown and become unusable. To prevent contamination keep all bottles and Petri dishes sealed until ready to use. • Pouring Plates • Materials Needed: • Draft-free enclosure or Laminar flow hood • 70% isopropyl alcohol • Petri dishes • Microwave or hot water bath or autoclave 1. Melt the agar using one of the following methods: a) Autoclave: Loosen the cap on the agar bottle and autoclave the bottle at 15 psi for five minutes. While wearing heat-protective gloves, carefully remove the hot bottle and let it cool to between 75–55°C before pouring. This takes approximately 15 minutes. b)Water Bath: Loosen the cap on the agar bottle and place it into a water bath. Water temperature should remain at around 100°C. Leave it in the water bath until the agar is completely melted. While wearing heat- protective gloves, carefully remove the hot bottle and let it cool to between 75–55°C before pouring. c) Microwave: Loosen the cap on the agar bottle before microwaving. -

Microlab® STAR™
Microlab ® STAR™ Microlab ® STAR ™ AUTOMATED WORKFLOW SOLUTIONS CENTERED AROUND YOUR ASSAY The STAR combines Hamilton's patented pipetting technology including precise lock-and-key tip attachment, unrivaled liquid level detection, and comprehensive volume ranges to create flexible liquid handling workstations. Available in three base platform sizes, the STAR portfolio incorporates countless options to automate your workflows. Hamilton Robotics has also partnered with top leaders in the biotechnology industry to provide Standard Solutions based on commonly automated applications. Offering ready-to-start protocols for a variety of applications such as NGS, ELISA, and forensic assays, our Standard Solutions provide a faster way to automate your processes. 2 1 PATENTED TECHNOLOGY The STAR utilizes Hamilton’s proprietary Compressed O-Ring Expansion (CO-RE®) technology. CO-RE minimizes the production of aerosols and allows disposable tips or washable, steel needles to be used on channels in the same run. 2 MULTI-FUNCTIONAL ARM Our technology offers high pipetting accuracy and precision, from sub-microliter to large volumes, using Independent Channels and/or the Multi-Probe Head (MPH). Labware transportation is possible with the iSWAP® or CO-RE Grippers. The STAR can incorporate a camera, tube transportation, and other channel tools on a single arm. Comprehensive pipetting range: ■■■0.5 μL to 1 mL using the 1 mL Independent Channel ■■■50 μL to 5 mL using the 5 mL Independent Channel ■■■1 μL to 1 mL using the CO-RE 96 MPH ■■■0.1 μL to 50 μL using the CO-RE 384 MPH 3 FLEXIBLE SETUP The high-capacity deck is customized specific to your workflow, accommodating a wide range of labware and automated devices that can easily be exchanged to support multiple assays on one platform. -

Restoring Bacteria-Impaired Waters a Toolkit to Help Local Stakeholders Identify and Eliminate Potential Pathogen Problems
Restoring Bacteria-Impaired Waters A Toolkit to Help Local Stakeholders Identify and Eliminate Potential Pathogen Problems developed by the Water Quality Restoration Program Division of Environmental Assessment and Restoration Florida Department of Environmental Protection Version 3.0, August 2018 2600 Blair Stone Road Tallahassee, FL 32399-2400 https://floridadep.gov Restoring Bacteria-Impaired Waters: A Toolkit to Help Local Stakeholders Identify and Eliminate Potential Pathogen Problems, July 2018 Table of Contents 1.0 Context ............................................................................................................................................. 4 1.1 Purpose ............................................................................................................................................. 4 1.2 Document Outline ............................................................................................................................ 5 1.3 DEP's Watershed Management Approach ................................................................................... 5 1.4 Coordinating with DEP ................................................................................................................... 6 1.5 Recommended Approach ................................................................................................................ 6 2.0 Understanding the Basin ............................................................................................................... 10 2.1 Causes of FIB Impairments -

Loading and Attenuation of Fecal Indicator Bacteria in Emigration Creek
LOADING AND ATTENUATION OF FECAL INDICATOR BACTERIA IN EMIGRATION CREEK by Garrick Douglas Willden A thesis submitted to the faculty of The University of Utah in partial fulfillment of the requirements for the degree of Master of Science in Civil Engineering Department of Civil and Environmental Engineering The University of Utah May 2006 i Copyright © Garrick Douglas Willden 2006 All Rights Reserved ii iii ABSTRACT A two-part study was performed in Emigration Creek from June to October 2005 to determine the warm season loading and concentration variability of fecal- indicator bacteria and investigate the fate and transport of bacteria within Emigration Creek. Emigration Creek is a small mountain stream draining a 47-km2 urbanizing drainage basin into the Salt Lake City metropolitan area. The warm season monitoring component of the protocol involved measuring streamflow and collecting weekly grab samples from eight locations in the creek for 16 weeks and analyzing for bacteriological and chemical fecal-indicators including total coliform, E. coli, enterococci, nitrate, chloride, and ammonia. Samples were collected and flows were measured twice daily to capture diurnal variation of the concentrations and bacteria flux (the number of bacteria passing each sampling site in time). The bacterial fate and transport component of the study involved introducing a bacteria tracer into an upstream reach of the creek in early October and collecting samples downstream to monitor the bacteria breakthrough curve over a period of 2 days. The results of the warm season monitoring indicated that most of the sampling sites exceeded the Utah numeric water quality criteria for E. coli (new bacteriological criterion, 206 No./100mL) from July to mid August. -

Comments on the US EPA “Review of Coliphages As Possible Indicators of Fecal Contamination for Ambient Water Quality”
Comments on the US EPA “Review of Coliphages as Possible Indicators of Fecal Contamination for Ambient Water Quality” Prepared for the National Association of Clean Water Agencies (NACWA) Samuel Dorevitch, MD, MPH University of Illinois at Chicago School of Public Health February 15, 2016 1 Executive Summary Millions of people each year enjoy using beaches, lakes, and rivers for recreation. Disease- causing microbes – pathogens – found in surface waters can present a threat to public health, particularly as a cause of gastrointestinal illness. Viral pathogens have been difficult, costly, and time-consuming to measure in surface waters. In order to address the challenges of 1) estimating the likelihood of pathogen presence and pathogen concentration in surface waters, and 2) estimating the health risks of surface water recreation, “indicators” have been monitored in surface waters as an alternative to pathogens. For decades, fecal indicator bacteria, such as E. coli and enterococci bacteria have been monitored in surface waters to satisfy a variety of Clean Water Act requirements. Coliphage viruses – viruses that infect E. coli bacteria – have been evaluated as indicators of wastewater treatment efficacy, human fecal pollution of surface waters, pathogenic virus presence in surface waters, and human health risk. In April, 2015, the US EPA Office of Water published a review of coliphage virus as a potential indicator of pathogens in surface wasters. That publication, “Review of Coliphages as Possible Indicators of Fecal Contamination for Ambient Water Quality,” included reviews of research studies that evaluated coliphage measurements as predictors of health risks of water recreation and pathogen presence. The present document, prepared for the National Association of Clean Water Agencies (NACWA), provides information from research papers that were not included in the EPA review as well as additional information from papers that were included in the EPA review. -

Food Protection Trends 2010-09: Vol 30 Iss 9
September 2010 ee Vol. a 30, No. 9 Periodicals 6200 Aurora Avenue ¢ Suite 200W Des Moines, lowa 50322-2864, USA merle Mm manele drele Mm igsalels Science and NewS trom the international Association for Food Protection Inter-agency Public Health Collaboration Pathogens Used with Biltong Product www.foodprotection.org BD |; Microbiology Media Solutions for Food Safety Reduce the risk to consumer safety with BBL Campy- e Over 170 years of combined Difco” and Cefex Agar* prepared plated medium for the isolation, BBL” microbiology experience enumeration and detection of Camplyobacter species directly from poultry. ' Microbiology— It’s what we do e Adopted by the National Advisory Committee on Microbiological Criteria for Foods for the isolation Find out what we can do for you. of Campylobacter species from chicken carcasses! Visit us on the web at www.bd.com/ds ¢ Better selectivity and easier differentiation of C. jejuni from other flora? BD Diagnostics 800.638.8663 *U.S. Patent No. 5,891,709 licensed to Becton, Dickinson and Company www.bd.com/ds 1 aa.) OAS A Food Process Control Microorganisms When the world’s leading food manufacturers need to meet expanding regulatory, liability, and quality assurance challenges they put their trust in Microbiologics. Our food process controls are guaranteed to deliver a specific number of colony forming units. Each easy-to-use microorganism preparation is authentic and traceable to an original reference strain. EZ-FPC™ * Qualitative preparations for Presence/Absence Testing * Quantitative preparations -

Evobot: an Open-Source, Modular, Liquid Handling Robot for Scientific Experiments
applied sciences Article EvoBot: An Open-Source, Modular, Liquid Handling Robot for Scientific Experiments Andres Faiña 1,* , Brian Nejati 2 and Kasper Stoy 1 1 Robots, Evolution and Art Lab (REAL), Department of Computer Science, IT University of Copenhagen, 2300 Copenhagen, Denmark; [email protected] 2 Department of Mechanical and Industrial Engineering, University of Toronto, Toronto, ON M5S 1A1, Canada; His work was carried out while he was employed at ITU; [email protected] * Correspondence: [email protected]; Tel.: +45-7218-5258 Received: 13 October 2019; Accepted: 17 January 2020; Published: 23 January 2020 Featured Application: Our modular liquid handling robot can be applied to automate scientific experiments in research labs, where tasks change often, and to carry out new kinds of experiments that cannot be done manually. Abstract: Commercial liquid handling robots are rarely appropriate when tasks change often, which is the case in the early stages of biochemical research. In order to address it, we have developed EvoBot, a liquid handling robot, which is open-source and employs a modular design. The combination of an open-source and a modular design is particularly powerful because functionality is divided into modules with simple, well-defined interfaces, hence customisation of modules is possible without detailed knowledge of the entire system. Furthermore, the modular design allows end-users to only produce and assemble the modules that are relevant for their specific application. Hence, time and money are not wasted on functionality that is not needed. Finally, modules can easily be reused. In this paper, we describe the EvoBot modular design and through scientific experiments such as basic liquid handling, nurturing of microbial fuel cells, and droplet chemotaxis experiments document how functionality is increased one module at a time with a significant amount of reuse. -

Process Skills Review Warm-Up C
Process Skills Review Warm-Up C. • Define function • Match the following 1. Puts out fire 2. Curved line of liquid in a graduated cylinder 3. Used to observe insects A. B. Which of the following describes the correct way to handle chemicals in a laboratory? A. It is safe to combine unknown chemicals as long as only small amounts are used. B. Return all chemicals to their original containers. C. Always pour extra amounts of the chemicals called for the experiment. D. To test for odors, always use a wafting motion. Which of the following describes the correct way to handle chemicals in a laboratory? A. It is safe to combine unknown chemicals as long as only small amounts are used. B. Return all chemicals to their original containers. C. Always pour extra amounts of the chemicals called for the experiment. D. To test for odors, always use a wafting motion. What task is being performed in the picture? A. measuring mass with a graduated cylinder B. measuring length with a triple beam balance C. measuring mass with a triple beam balance D. measuring length with a metric ruler What task is being performed in the picture? A. measuring mass with a graduated cylinder B. measuring length with a triple beam balance C. measuring mass with a triple beam balance D. measuring length with a metric ruler John and Lisa are conducting an experiment in their 7th grade science class in which they are handling potentially dangerous chemicals. As John is pouring a chemical from a beaker to a graduated cylinder, he splashes some of the chemical into his eyes. -

Issue Paper: Revisions to the Water Quality Standard for Bacteria
Issue Paper: Revisions to the Water Quality Standard for Bacteria By: Aron Borok August 2016 Environmental Solutions/ Standards and Assessment 811 SW 6th Avenue Portland, OR 97204 Phone: 503-229-5050 800-452-4011 Fax: 503-229-5850 Contact: Aron Borok www.oregon.gov/DEQ DEQ is a leader in restoring, maintaining and enhancing the quality of Oregon’s air, land and water. Oregon Department of Environmental Quality Last updated: 8/22/16 By: Aron Borok This report prepared by: Oregon Department of Environmental Quality 811 SW 6th Avenue Portland, OR 97204 1-800-452-4011 www.oregon.gov/deq Contact: Aron Borok 503-229-5050 Documents can be provided upon request in an alternate format for individuals with disabilities or in a language other than English for people with limited English skills. To request a document in another format or language, call DEQ in Portland at 503-229-5696, or toll-free in Oregon at 1-800-452-4011, ext. 5696; or email [email protected]. State of Oregon Department of Environmental Quality ii I would like to acknowledge Debra Sturdevant for providing many excellent comments for this report and the project in general. I also would like to thank James McConnaghie for preparing draft designated use maps, as well as initial maps detailing shellfishing areas and salinity data. I also would like to acknowledge staff from the Oregon Department of Fish and Wildlife and Oregon Department of Agriculture Shellfish Programs and the Environmental Protection Agency Western Ecology Division for providing information and support related to shellfish distribution in Oregon.