Termites and Ecosystem Function - Og Desouza, Eliana Marques Cancello
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Evolution and Ecology of Termite Nesting Behavior and Its Impact On
1 Evolution and Ecology of Termite Nesting Behavior and Its Impact on Disease Susceptibility A dissertation presented by Marielle Aimée Postava-Davignon to The Department of Biology In partial fulfillment of the requirements for the degree of Doctor of Philosophy in the field of Biology Northeastern University Boston, Massachusetts April, 2010 2 Evolution and Ecology of Termite Nesting Behavior and Its Impact on Disease Susceptibility by Marielle Aimée Postava-Davignon ABSTRACT OF DISSERTATION Submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in Biology in the Graduate School of Arts and Sciences of Northeastern University, April, 2010 3 Abstract Termites construct nests that are often structurally species-specific. They exhibit a high diversity of nest structures, but their nest evolution is largely unknown. Current hypotheses for the factors that influenced nest evolution include adaptations that improved nest thermoregulation, defense against predators, and competition for limited nest sites. Studies have shown a lower prevalence of pathogens and parasites in arboreal nesting animal species compared to ground nesters. Nest building behavior is plastic and can adapt to changing environments. As termites can detect and avoid pathogens, I hypothesized that the evolution of arboreal termite nests was an adaptation to avoid infection. To test this, bacteria and fungi from nest cores, trails, and surrounding soils of the arboreal nesting Nasutitermes acajutlae were cultured. Abiotic factors such as temperature, relative humidity, and light were measured to elucidate how they influenced the interactions between termites and microbes. Fungi associated with N. acajutlae were identified to determine the potential pathogenic pressures these termites encounter in their nest as compared to the external environment. -

In Termite Nests (Blattodea: Termitidae) in a Cocoa Plantation in Brazil Biota Neotropica, Vol
Biota Neotropica ISSN: 1676-0611 [email protected] Instituto Virtual da Biodiversidade Brasil Teixeira Lisboa, Jonathas; Guerreiro Couto, Erminda da Conceição; Pereira Santos, Pollyanna; Charles Delabie, Jacques Hubert; Araujo, Paula Beatriz Terrestrial isopods (Crustacea: Isopoda: Oniscidea) in termite nests (Blattodea: Termitidae) in a cocoa plantation in Brazil Biota Neotropica, vol. 13, núm. 3, julio-septiembre, 2013, pp. 393-397 Instituto Virtual da Biodiversidade Campinas, Brasil Available in: http://www.redalyc.org/articulo.oa?id=199128991039 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative Biota Neotrop., vol. 13, no. 3 Terrestrial isopods (Crustacea: Isopoda: Oniscidea) in termite nests (Blattodea: Termitidae) in a cocoa plantation in Brazil Jonathas Teixeira Lisboa1,7, Erminda da Conceição Guerreiro Couto2, Pollyanna Pereira Santos3, Jacques Hubert Charles Delabie4,5 & Paula Beatriz Araujo6 1Universidade Estadual de Santa Cruz – UESC, Campus Soane Nazaré de Andrade, Rod. Ilhéus-Itabuna, km 16, CEP 45662-900, Ilhéus, BA, Brasil. www.uesc.br/zoologia 2Universidade Estadual de Santa Cruz – UESC, Campus Soane Nazaré de Andrade, Rod. Ilhéus-Itabuna, km 16, CEP 45662-900, Ilhéus, BA, Brasil. www.uesc.br/cursos/pos_graduacao/mestrado/ppsat 3Universidade Federal de Viçosa – UFV, CEP 36570-000 Viçosa, MG, Brasil. www.pos.entomologia.ufv.br 4Departamento de Ciências Agrárias e Ambientais, Universidade Estadual de Santa Cruz – UESC, Campus Soane Nazaré de Andrade, Rod. Ilhéus-Itabuna, km 16, CEP 45662-900, Ilhéus, BA, Brasil. www.uesc.br/dcaa/index.php 5Laboratório de Mirmecologia, Convênio UESC/CEPLAC, Centro de Pesquisa do Cacau, CP 7, CEP 45600-000 Itabuna, BA, Brasil. -

AUSTRALIAN TERMITOPHILES ASSOCIATED with MICROCEROTERMES (Isoptera: Amitermitinae) I
Pacific Insects 12 (1): 9-15 20 May 1970 AUSTRALIAN TERMITOPHILES ASSOCIATED WITH MICROCEROTERMES (Isoptera: Amitermitinae) I. A new Subtribe, genus, and species (Coleoptera, Staphylinidae) with notes on their behavior1 By David H. Kistner2 Abstract: A new Subtribe (Microceroxenina) of the tribe Athetini is described. The single included genus and species (both new) is Microceroxenus alzadae which was cap tured with Microcerotermes turneri in North Queensland. Behavioral observations are presented which support the interpretation that Microceroxenus is well-integrated into the social life of the termites. Observations of the release of alates by the host ter mites are presented which support the interpretation that the release of alates in these termites is simultaneous among colonies in a given area, is of short duration, and oc curs rather infrequently. Not many species of termitophiles have been found with termites of the genus Micro cerotermes Silvestri (Amitermitinae) or even from the genera related to Microcerotermes such as Amphidotermes or Globitermes (Ahmad 1950). Only 1 species of staphylinid has been previously recorded and that species is Termitochara kraatzi Wasmann which was collected with Microcerotermes sikorae (Wasmann) from Madagascar (Seevers 1957). The same species of termitophile has also been recorded from a nest of Capritermes capricor- nis (Wasmann), which belongs to an entirely different subfamily (Termitinae), by Was mann (1893). No one really believes either of these termites is the true host of the species as the nearest relatives of Termitochara are found principally with the Nasutiter- mitinae. It was therefore a real pleasure to open up a Microcerotermes nest and find numerous staphylinids there, particularly when opening up nests of the same genus in Africa had never yielded any staphylinids. -

Ant‐Termite Interactions
Biol. Rev. (2020), 95, pp. 555–572. 555 doi: 10.1111/brv.12577 Ant-termite interactions: an important but under-explored ecological linkage Jiri Tuma1,2,3* , Paul Eggleton4 and Tom M. Fayle1,5 1Biology Centre of the Czech Academy of Sciences, Institute of Entomology, Ceske Budejovice, Czech Republic 2Biology Centre of the Czech Academy of Sciences, Institute of Soil Biology, Ceske Budejovice, Czech Republic 3Faculty of Science, University of South Bohemia, Ceske Budejovice, Czech Republic 4Life Sciences Department, Natural History Museum, London, UK 5Institute for Tropical Biology and Conservation, Universiti Malaysia Sabah, Kota Kinabalu, Malaysia ABSTRACT Animal interactions play an important role in understanding ecological processes. The nature and intensity of these inter- actions can shape the impacts of organisms on their environment. Because ants and termites, with their high biomass and range of ecological functions, have considerable effects on their environment, the interaction between them is important for ecosystem processes. Although the manner in which ants and termites interact is becoming increasingly well studied, there has been no synthesis to date of the available literature. Here we review and synthesise all existing literature on ant– termite interactions. We infer that ant predation on termites is the most important, most widespread, and most studied type of interaction. Predatory ant species can regulate termite populations and subsequently slow down the decomposi- tion of wood, litter and soil organic matter. As a consequence they also affect plant growth and distribution, nutrient cycling and nutrient availability. Although some ant species are specialised termite predators, there is probably a high level of opportunistic predation by generalist ant species, and hence their impact on ecosystem processes that termites are known to provide varies at the species level. -

Isoptera) in New Guinea 55 Doi: 10.3897/Zookeys.148.1826 Research Article Launched to Accelerate Biodiversity Research
A peer-reviewed open-access journal ZooKeys 148: 55–103Revision (2011) of the termite family Rhinotermitidae (Isoptera) in New Guinea 55 doi: 10.3897/zookeys.148.1826 RESEARCH ARTICLE www.zookeys.org Launched to accelerate biodiversity research Revision of the termite family Rhinotermitidae (Isoptera) in New Guinea Thomas Bourguignon1,2,†, Yves Roisin1,‡ 1 Evolutionary Biology and Ecology, CP 160/12, Université Libre de Bruxelles (ULB), Avenue F.D. Roosevelt 50, B-1050 Brussels, Belgium 2 Present address: Graduate School of Environmental Science, Hokkaido Uni- versity, Sapporo 060–0810, Japan † urn:lsid:zoobank.org:author:E269AB62-AC42-4CE9-8E8B-198459078781 ‡ urn:lsid:zoobank.org:author:73DD15F4-6D52-43CD-8E1A-08AB8DDB15FC Corresponding author: Yves Roisin ([email protected]) Academic editor: M. Engel | Received 19 July 2011 | Accepted 28 September 2011 | Published 21 November 2011 urn:lsid:zoobank.org:pub:27B381D6-96F5-482D-B82C-2DFA98DA6814 Citation: Bourguignon T, Roisin Y (2011) Revision of the termite family Rhinotermitidae (Isoptera) in New Guinea. In: Engel MS (Ed) Contributions Celebrating Kumar Krishna. ZooKeys 148: 55–103. doi: 10.3897/zookeys.148.1826 Abstract Recently, we completed a revision of the Termitidae from New Guinea and neighboring islands, record- ing a total of 45 species. Here, we revise a second family, the Rhinotermitidae, to progress towards a full picture of the termite diversity in New Guinea. Altogether, 6 genera and 15 species are recorded, among which two species, Coptotermes gambrinus and Parrhinotermes barbatus, are new to science. The genus Heterotermes is reported from New Guinea for the first time, with two species restricted to the southern part of the island. -

Feeding and Foraging Behaviors of Subterranean Termites (Isoptera:Rhinotermitidae)
Louisiana State University LSU Digital Commons LSU Historical Dissertations and Theses Graduate School 1989 Feeding and Foraging Behaviors of Subterranean Termites (Isoptera:Rhinotermitidae). Keith Scott elD aplane Louisiana State University and Agricultural & Mechanical College Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_disstheses Recommended Citation Delaplane, Keith Scott, "Feeding and Foraging Behaviors of Subterranean Termites (Isoptera:Rhinotermitidae)." (1989). LSU Historical Dissertations and Theses. 4838. https://digitalcommons.lsu.edu/gradschool_disstheses/4838 This Dissertation is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Historical Dissertations and Theses by an authorized administrator of LSU Digital Commons. For more information, please contact [email protected]. INFORMATION TO USERS The most advanced technology has been used to photograph and reproduce this manuscript from the microfilm master. UMI films the text directly from the original or copy submitted. Thus, some thesis and dissertation copies are in typewriter face, while others may be from any type of computer printer. The quality of this reproduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleedthrough, substandard margins, and improper alignment can adversely afreet reproduction. In the unlikely event that the author did not send UMI a complete manuscript and there are missing pages, these will be noted. Also, if unauthorized copyright material had to be removed, a note will indicate the deletion. Oversize materials (e.g., maps, drawings, charts) are reproduced by sectioning the original, beginning at the upper left-hand corner and continuing from left to right in equal sections with small overlaps. -

The Termite by Ogden Nash
Biological studies on two European termite species: establishment risk in the UK Laetitia Virginie Laine B.Sc. M.Sc. D.I.C. A thesis submitted for the degree of Doctor of Philosophy of the University of London November 2002 Department of Biological Sciences, Imperial College, Silwood Park, Ascot, SL5 7PY, Berkshire Abstract The discovery of an accidental introduction of termites into Devon in 1994 generated great interest as termites were previously thought to be unable to establish in the UK due to unfavourable climatic conditions. Information about the species present in Devon, Reticulitermes grassei, was found to be lacking and the present study was undertaken to determine the importance of various abiotic and biotic factors in establishment of this species. The factors included in the study were the minimum termite number for establishment, the consumption of wood and its effect on survival and temperature and soil type. A review of the literature was also conducted, detailing the problems with the taxonomy of this termite genus, their present distribution pattern and the life cycle of Reticulitermes species. Two populations of both R. grassei and R. santonensis were studied. The effect of the minimum termite number was found to be significant in both laboratory and field conditions. However, survival decreased in the laboratory and increased in the field with increased number of termites. Consumption experiments were performed using blocks of Scots pine, beech and oak. In most cases termite populations were found to consume and survive best on oak. Consumption was also tested on live seedlings but these results were inconclusive. Survival was observed to increase with increased temperature. -
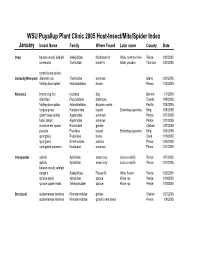
Host Insect List 2005
WSU Puyallup Plant Clinic 2005 Host-Insect/Mite/Spider Index January Insect Name Family Where Found Latin name County Date Crop balsam woolly adelgid Adelgididae Nordmann fir Abies nordmannian Pierce 1/31/2005 coneworm Tortricidae noble fir Abies procera Thurston 1/31/2005 comb footed spider, Curiosity/Non-pest Steatoda sp. Theridiidae unknown Island 1/21/2005 folding-door spider Antrodiaetidae house Pierce 1/12/2005 Nuisance brown dog tick Ixodidae dog Benton 1/7/2005 drainflies Psychodidae bathroom Cowlitz 1/28/2005 folding-door spider Antrodiaetidae daycare center Pacific 1/24/2005 fungus gnats Fungivoridae loquat Eriobotrya japonica King 1/24/2005 giant house spider Agelenidae unknown Pierce 1/21/2005 hobo spider Agelenidae unknown Pierce 1/21/2005 moisture ant queen Formicidae garden Clallam 1/27/2005 psocids Psocidae loquat Eriobotrya japonica King 1/24/2005 springtails Poduridae home Clark 1/10/2005 springtails Sminthuridae outside Pierce 1/26/2005 variegated cutworm Noctuidae unknown Pierce 1/21/2005 Ornamental aphids Aphididae sweet bay Laurus nobilis Pierce 1/27/2005 aphids Aphididae sweet bay Laurus nobilis Pierce 1/27/2005 balsam woolly adelgid nymphs Adelgididae Frasier fir Abies fraseri Pierce 1/25/2005 spruce aphid Aphididae spruce Picea sp. Pierce 1/10/2005 spruce spider mites Tetranchyidae spruce Picea sp. Pierce 1/10/2005 Structural subterranean termites Rhinotermitidae garden Clallam 1/27/2005 subterranean termites Rhinotermitidae ground near shed Pierce 1/8/2005 February Insect Name Family Where Found Latin name County -

Isoptera Book Chapter
Isoptera 535 See Also the Following Articles Biodiversity ■ Biogeographical Patterns ■ Cave Insects ■ Introduced Insects Further Reading Carlquist , S. ( 1974 ) . “ Island Biology . ” Columbia University Press , New York and London . Gillespie , R. G. , and Roderick , G. K. ( 2002 ) . Arthropods on islands: Colonization, speciation, and conservation . Annu. Rev. Entomol. 47 , 595 – 632 . Gillespie , R. G. , and Clague , D. A. (eds.) (2009 ) . “ Encyclopedia of Islands. ” University of California Press , Berkeley, CA . Howarth , F. G. , and Mull , W. P. ( 1992 ) . “ Hawaiian Insects and Their Kin . ” University of Hawaii Press , Honolulu, HI . MacArthur , R. H. , and Wilson , E. O. ( 1967 ) . “ The Theory of Island Biogeography . ” Princeton University Press , Princeton, NJ . Wagner , W. L. , and Funk , V. (eds.) ( 1995 ) . “ Hawaiian Biogeography Evolution on a Hot Spot Archipelago. ” Smithsonian Institution Press , Washington, DC . Whittaker , R. J. , and Fern á ndez-Palacios , J. M. ( 2007 ) . “ Island Biogeography: Ecology, Evolution, and Conservation , ” 2nd ed. Oxford University Press , Oxford, U.K . I Isoptera (Termites) Vernard R. Lewis FIGURE 1 Castes for Isoptera. A lower termite group, University of California, Berkeley Reticulitermes, is represented. A large queen is depicted in the center. A king is to the left of the queen. A worker and soldier are he ordinal name Isoptera is of Greek origin and refers to below. (Adapted, with permission from Aventis Environmental the two pairs of straight and very similar wings that termites Science, from The Mallis Handbook of Pest Control, 1997.) Thave as reproductive adults. Termites are small and white to tan or sometimes black. They are sometimes called “ white ants ” and can be confused with true ants (Hymenoptera). -

Stable Isotope Methods in Biological and Ecological Studies of Arthropods
eea_572.fm Page 3 Tuesday, June 12, 2007 4:17 PM DOI: 10.1111/j.1570-7458.2007.00572.x Blackwell Publishing Ltd MINI REVIEW Stable isotope methods in biological and ecological studies of arthropods CORE Rebecca Hood-Nowotny1* & Bart G. J. Knols1,2 Metadata, citation and similar papers at core.ac.uk Provided by Wageningen University & Research Publications 1International Atomic Energy Agency (IAEA), Agency’s Laboratories Seibersdorf, A-2444 Seibersdorf, Austria, 2Laboratory of Entomology, Wageningen University and Research Centre, P.O. Box 8031, 6700 EH Wageningen, The Netherlands Accepted: 13 February 2007 Key words: marking, labelling, enrichment, natural abundance, resource turnover, 13-carbon, 15-nitrogen, 18-oxygen, deuterium, mass spectrometry Abstract This is an eclectic review and analysis of contemporary and promising stable isotope methodologies to study the biology and ecology of arthropods. It is augmented with literature from other disciplines, indicative of the potential for knowledge transfer. It is demonstrated that stable isotopes can be used to understand fundamental processes in the biology and ecology of arthropods, which range from nutrition and resource allocation to dispersal, food-web structure, predation, etc. It is concluded that falling costs and reduced complexity of isotope analysis, besides the emergence of new analytical methods, are likely to improve access to isotope technology for arthropod studies still further. Stable isotopes pose no environmental threat and do not change the chemistry or biology of the target organism or system. These therefore represent ideal tracers for field and ecophysiological studies, thereby avoiding reductionist experimentation and encouraging more holistic approaches. Con- sidering (i) the ease with which insects and other arthropods can be marked, (ii) minimal impact of the label on their behaviour, physiology, and ecology, and (iii) environmental safety, we advocate more widespread application of stable isotope technology in arthropod studies and present a variety of potential uses. -

Diversity of Ants and Termites of the Botanical Garden of the University of Lomé, Togo
insects Article Diversity of Ants and Termites of the Botanical Garden of the University of Lomé, Togo Boris Dodji Kasseney 1,* , Titati Bassouo N’tie 1, Yaovi Nuto 1, Dekoninck Wouter 2 , Kolo Yeo 3 and Isabelle Adolé Glitho 1 1 Laboratoire d’Entomologie Appliquée, Département de Zoologie et de Biologie Animale, Université de Lomé, 01 BP 1515, Lomé 01, Lome 151, Togo 2 RBINS Scientific Service Heritage/O.D. Taxonomy and Phylogeny, Curator Entomology Collections, Vautierstraat 29, 1000 Brussels, Belgium 3 Université Nangui Abrogoua, 02 BP 801 Abidjan 02, Abidjan, Côte d’Ivoire * Correspondence: [email protected]; Tel.: +228-906-156-74 Received: 6 June 2019; Accepted: 22 July 2019; Published: 23 July 2019 Abstract: Ants and termites are used as bioindicators in many ecosystems. Little knowledge is available about them in Togo, especially ants. This study aimed to find out how ants and termites could be used to assess the restoration of former agricultural land. These insect groups were sampled within six transects of 50 2 m2 (using pitfall traps, monoliths, baits for ants and hand sampling for × termites) in two consecutive habitats: open area (grassland) and covered area (an artificial forest). Seventeen termite species and 43 ant species were collected. Seven ant species were specific to the covered area against four for the open area, while four unshared species of termite were found in the open area against three in the covered area. The presence of unshared species was linked to vegetation, as Trinervitermes (Holmgren, 1912), a grass feeding termite, was solely found in open area. Also, for some ant species like Cataulacus traegaordhi (Santschi, 1914), Crematogaster (Lund, 1831) species, Oecophylla longinoda (Latreille, 1802) and Tetraponera mocquerysi (Brown, 1960), all arboreal species, vegetation was a determining factor for their presence. -

Evaluation of the Chemical Defense Fluids of Macrotermes Carbonarius
www.nature.com/scientificreports OPEN Evaluation of the chemical defense fuids of Macrotermes carbonarius and Globitermes sulphureus as possible household repellents and insecticides S. Appalasamy1,2*, M. H. Alia Diyana2, N. Arumugam2 & J. G. Boon3 The use of chemical insecticides has had many adverse efects. This study reports a novel perspective on the application of insect-based compounds to repel and eradicate other insects in a controlled environment. In this work, defense fuid was shown to be a repellent and insecticide against termites and cockroaches and was analyzed using gas chromatography-mass spectrometry (GC– MS). Globitermes sulphureus extract at 20 mg/ml showed the highest repellency for seven days against Macrotermes gilvus and for thirty days against Periplaneta americana. In terms of toxicity, G. sulphureus extract had a low LC50 compared to M. carbonarius extract against M. gilvus. Gas chromatography–mass spectrometry analysis of the M. carbonarius extract indicated the presence of six insecticidal and two repellent compounds in the extract, whereas the G. sulphureus extract contained fve insecticidal and three repellent compounds. The most obvious fnding was that G. sulphureus defense fuid had higher potential as a natural repellent and termiticide than the M. carbonarius extract. Both defense fuids can play a role as alternatives in the search for new, sustainable, natural repellents and termiticides. Our results demonstrate the potential use of termite defense fuid for pest management, providing repellent and insecticidal activities comparable to those of other green repellent and termiticidal commercial products. A termite infestation could be silent, but termites are known as destructive urban pests that cause structural damage by infesting wooden and timber structures, leading to economic loss.