Temporal and Spatial Movement Patterns of Striped Bass in the Minas Passage, Bay of Fundy
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Barriers to Fish Passage in Nova Scotia the Evolution of Water Control Barriers in Nova Scotia’S Watershed
Dalhousie University- Environmental Science Barriers to Fish Passage in Nova Scotia The Evolution of Water Control Barriers in Nova Scotia’s Watershed By: Gillian Fielding Supervisor: Shannon Sterling Submitted for ENVS 4901- Environmental Science Honours Abstract Loss of connectivity throughout river systems is one of the most serious effects dams impose on migrating fish species. I examine the extent and dates of aquatic habitat loss due to dam construction in two key salmon regions in Nova Scotia: Inner Bay of Fundy (IBoF) and the Southern Uplands (SU). This work is possible due to the recent progress in the water control structure inventory for the province of Nova Scotia (NSWCD) by Nova Scotia Environment. Findings indicate that 586 dams have been documented in the NSWCD inventory for the entire province. The most common main purpose of dams built throughout Nova Scotia is for hydropower production (21%) and only 14% of dams in the database contain associated fish passage technology. Findings indicate that the SU is impacted by 279 dams, resulting in an upstream habitat loss of 3,008 km of stream length, equivalent to 9.28% of the total stream length within the SU. The most extensive amount of loss occurred from 1920-1930. The IBoF was found to have 131 dams resulting in an upstream habitat loss of 1, 299 km of stream length, equivalent to 7.1% of total stream length. The most extensive amount of upstream habitat loss occurred from 1930-1940. I also examined if given what I have learned about the locations and dates of dam installations, are existent fish population data sufficient to assess the impacts of dams on the IBoF and SU Atlantic salmon populations in Nova Scotia? Results indicate that dams have caused a widespread upstream loss of freshwater habitat in Nova Scotia howeverfish population data do not exist to examine the direct impact of dam construction on the IBoF and SU Atlantic salmon populations in Nova Scotia. -

Flood Frequency Analyses for New Brunswick Rivers Canadian Technical Report of Fisheries and Aquatic Sciences 2920
Flood Frequency Analyses for New Brunswick Rivers Aucoin, F., D. Caissie, N. El-Jabi and N. Turkkan Department of Fisheries and Oceans Gulf Region Oceans and Science Branch Diadromous Fish Section P.O. Box 5030, Moncton, NB, E1C 9B6 2011 Canadian Technical Report of Fisheries and Aquatic Sciences 2920 Canadian Technical Report of Fisheries and Aquatic Sciences Technical reports contain scientific and technical information that contributes to existing knowledge but which is not normally appropriate for primary literature. Technical reports are directed primarily toward a worldwide audience and have an international distribution. No restriction is placed on subject matter and the series reflects the broad interests and policies of Fisheries and Oceans, namely, fisheries and aquatic sciences. Technical reports may be cited as full publications. The correct citation appears above the abstract of each report. Each report is abstracted in the data base Aquatic Sciences and Fisheries Abstracts. Technical reports are produced regionally but are numbered nationally. Requests for individual reports will be filled by the issuing establishment listed on the front cover and title page. Numbers 1-456 in this series were issued as Technical Reports of the Fisheries Research Board of Canada. Numbers 457-714 were issued as Department of the Environment, Fisheries and Marine Service, Research and Development Directorate Technical Reports. Numbers 715-924 were issued as Department of Fisheries and Environment, Fisheries and Marine Service Technical Reports. The current series name was changed with report number 925. Rapport technique canadien des sciences halieutiques et aquatiques Les rapports techniques contiennent des renseignements scientifiques et techniques qui constituent une contribution aux connaissances actuelles, mais qui ne sont pas normalement appropriés pour la publication dans un journal scientifique. -

Striped Bass Morone Saxatilis
COSEWIC Assessment and Status Report on the Striped Bass Morone saxatilis in Canada Southern Gulf of St. Lawrence Population St. Lawrence Estuary Population Bay of Fundy Population SOUTHERN GULF OF ST. LAWRENCE POPULATION - THREATENED ST. LAWRENCE ESTUARY POPULATION - EXTIRPATED BAY OF FUNDY POPULATION - THREATENED 2004 COSEWIC COSEPAC COMMITTEE ON THE STATUS OF COMITÉ SUR LA SITUATION ENDANGERED WILDLIFE DES ESPÈCES EN PÉRIL IN CANADA AU CANADA COSEWIC status reports are working documents used in assigning the status of wildlife species suspected of being at risk. This report may be cited as follows: COSEWIC 2004. COSEWIC assessment and status report on the Striped Bass Morone saxatilis in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. vii + 43 pp. (www.sararegistry.gc.ca/status/status_e.cfm) Production note: COSEWIC would like to acknowledge Jean Robitaille for writing the status report on the Striped Bass Morone saxatilis prepared under contract with Environment Canada, overseen and edited by Claude Renaud the COSEWIC Freshwater Fish Species Specialist Subcommittee Co-chair. For additional copies contact: COSEWIC Secretariat c/o Canadian Wildlife Service Environment Canada Ottawa, ON K1A 0H3 Tel.: (819) 997-4991 / (819) 953-3215 Fax: (819) 994-3684 E-mail: COSEWIC/[email protected] http://www.cosewic.gc.ca Ếgalement disponible en français sous le titre Ếvaluation et Rapport de situation du COSEPAC sur la situation de bar rayé (Morone saxatilis) au Canada. Cover illustration: Striped Bass — Drawing from Scott and Crossman, 1973. Her Majesty the Queen in Right of Canada 2004 Catalogue No. CW69-14/421-2005E-PDF ISBN 0-662-39840-8 HTML: CW69-14/421-2005E-HTML 0-662-39841-6 Recycled paper COSEWIC Assessment Summary Assessment Summary – November 2004 Common name Striped Bass (Southern Gulf of St. -

A Review of Ice and Tide Observations in the Bay of Fundy
A tlantic Geology 195 A review of ice and tide observations in the Bay of Fundy ConDesplanque1 and David J. Mossman2 127 Harding Avenue, Amherst, Nova Scotia B4H 2A8, Canada departm ent of Physics, Engineering and Geoscience, Mount Allison University, 67 York Street, Sackville, New Brunswick E4L 1E6, Canada Date Received April 27, 1998 Date Accepted December 15,1998 Vigorous quasi-equilibrium conditions characterize interactions between land and sea in macrotidal regions. Ephemeral on the scale of geologic time, estuaries around the Bay of Fundy progressively infill with sediments as eustatic sea level rises, forcing fringing salt marshes to form and reform at successively higher levels. Although closely linked to a regime of tides with large amplitude and strong tidal currents, salt marshes near the Bay of Fundy rarely experience overflow. Built up to a level about 1.2 m lower than the highest astronomical tide, only very large tides are able to cover the marshes with a significant depth of water. Peak tides arrive in sets at periods of 7 months, 4.53 years and 18.03 years. Consequently, for months on end, no tidal flooding of the marshes occurs. Most salt marshes are raised to the level of the average tide of the 18-year cycle. The number of tides that can exceed a certain elevation in any given year depends on whether the three main tide-generating factors peak at the same time. Marigrams constructed for the Shubenacadie and Cornwallis river estuaries, Nova Scotia, illustrate how the estuarine tidal wave is reshaped over its course, to form bores, and varies in its sediment-carrying and erosional capacity as a result of changing water-surface gradients. -

Non-Resident Guide Exemption Application Form
NON-RESIDENT GUIDE EXEMPTION APPLICATION FORM STEP 1 – Provide your personal information (permanent address) Surname First Name Middle Initial Street Town/City Province/State Postal Code/Zip Code Country Telephone (Work) (Home) Fax (Work) Cellular Telephone (Home) E-Mail Address Note: In case of joint ownership, attach individual applications – maximum 5 per property STEP 2 – Provide information for exemption of individual(s) (if applicable) 1) Surname First Name Middle Initial Relationship to applicant 2) Surname First Name Middle Initial Relationship to applicant 3) Surname First Name Middle Initial Relationship to applicant Note: Only one individual at a time may accompany the applicant while angling/hunting. If you require more space, please attach additional sheet with immediate family member(s) information. STEP 3 – Provide New Brunswick property information Property Tax Code Number Assessed Value of Property Building(s) Description New Brunswick Address Note: You must attach a photocopy of your current New Brunswick Real Property Assessment & Tax Notice STEP 4 – Indicate angling and/or hunting information Angling - Exemption Request Indicate name of river(s) and section(s). Hunting - Exemption Request Indicate Wildlife Management Zone(s). Note: Please refer to Guide Required Waters and Wildlife Management Zones Map information sheets 60-6392E (10/18) STEP 5 – Indicate your application method Option A Option B Option C Application by mail Application by fax Application in person at ERD, Fish & Wildlife Branch STEP 6 – Indicate your payment method Annual fee of $150 Canadian Funds (no tax) Check only one box. Cash Cheque Money Order Visa MasterCard Note: Do not send cash by mail. Please make cheque or money order payable in the amount of $150 Canadian Funds to the Minister of Finance, Province of New Brunswick. -

C S a S S C C S
C S A S S C C S Canadian Science Advisory Secretariat Secrétariat canadien de consultation scientifique Research Document 2006/041 Document de recherche 2006/041 Not to be cited without Ne pas citer sans permission of the authors * autorisation des auteurs * Assessment of Status and Recovery Évaluation de la situation et du Potential for Striped bass (Morone potentiel de rétablissement du bar saxatilis) in the Southern Gulf of St. rayé (Morone saxatilis) dans le sud du Lawrence golfe du Saint-Laurent S.G. Douglas, G. Chaput and D. Caissie Department of Fisheries and Oceans Oceans and Science Branch, Gulf Region P.O. Box 5030 Moncton, New Brunswick E1C 9B6 * This series documents the scientific basis for the * La présente série documente les bases evaluation of fisheries resources in Canada. As scientifiques des évaluations des ressources such, it addresses the issues of the day in the halieutiques du Canada. Elle traite des time frames required and the documents it problèmes courants selon les échéanciers contains are not intended as definitive statements dictés. Les documents qu’elle contient ne on the subjects addressed but rather as progress doivent pas être considérés comme des énoncés reports on ongoing investigations. définitifs sur les sujets traités, mais plutôt comme des rapports d’étape sur les études en cours. Research documents are produced in the official Les documents de recherche sont publiés dans language in which they are provided to the la langue officielle utilisée dans le manuscrit Secretariat. envoyé au Secrétariat. This document is available on the Internet at: Ce document est disponible sur l’Internet à: http://www.dfo-mpo.gc.ca/csas/ ISSN 1499-3848 (Printed / Imprimé) © Her Majesty the Queen in Right of Canada, 2006 © Sa majesté la Reine, Chef du Canada, 2006 ii TABLE OF CONTENTS ABSTRACT.................................................................................................................................. -
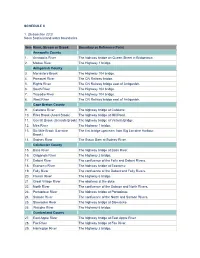
Nova Scotia Inland Water Boundaries Item River, Stream Or Brook
SCHEDULE II 1. (Subsection 2(1)) Nova Scotia inland water boundaries Item River, Stream or Brook Boundary or Reference Point Annapolis County 1. Annapolis River The highway bridge on Queen Street in Bridgetown. 2. Moose River The Highway 1 bridge. Antigonish County 3. Monastery Brook The Highway 104 bridge. 4. Pomquet River The CN Railway bridge. 5. Rights River The CN Railway bridge east of Antigonish. 6. South River The Highway 104 bridge. 7. Tracadie River The Highway 104 bridge. 8. West River The CN Railway bridge east of Antigonish. Cape Breton County 9. Catalone River The highway bridge at Catalone. 10. Fifes Brook (Aconi Brook) The highway bridge at Mill Pond. 11. Gerratt Brook (Gerards Brook) The highway bridge at Victoria Bridge. 12. Mira River The Highway 1 bridge. 13. Six Mile Brook (Lorraine The first bridge upstream from Big Lorraine Harbour. Brook) 14. Sydney River The Sysco Dam at Sydney River. Colchester County 15. Bass River The highway bridge at Bass River. 16. Chiganois River The Highway 2 bridge. 17. Debert River The confluence of the Folly and Debert Rivers. 18. Economy River The highway bridge at Economy. 19. Folly River The confluence of the Debert and Folly Rivers. 20. French River The Highway 6 bridge. 21. Great Village River The aboiteau at the dyke. 22. North River The confluence of the Salmon and North Rivers. 23. Portapique River The highway bridge at Portapique. 24. Salmon River The confluence of the North and Salmon Rivers. 25. Stewiacke River The highway bridge at Stewiacke. 26. Waughs River The Highway 6 bridge. -

Fishery Bulletin of the Fish and Wildlife Service V.53
'I', . FISRES OF '!'RE GULF OF MAINE. 101 Description.-The hickory shad differs rather Bay, though it is found in practically all of them. noticeably from the sea herring in that the point This opens the interesting possibility that the of origin of its dorsal fin is considerably in front of "green" fish found in Chesapeake Bay, leave the the mid-length of its trunk; in its deep belly (a Bay, perhaps to spawn in salt water.65 hickory shad 13~ in. long is about 4 in. deep but a General range.-Atlantic coast of North America herring of that length is only 3 in. deep) ; in the fact from the Bay of Fundy to Florida. that its outline tapers toward both snout and tail Occurrence in the Gulf oj Maine.-The hickory in side view (fig. 15); and in that its lower jaw shad is a southern fish, with the Gulf of Maine as projects farther beyond the upper when its mouth the extreme northern limit to its range. It is is closed; also, by the saw-toothed edge of its belly. recorded in scientific literature only at North Also, it lacks the cluster of teeth on the roof of the· Truro; at Provincetown; at Brewster; in Boston mouth that is characteristic of the herring. One Harbor; off Portland; in Casco Ba3T; and from the is more likely to confuse a hickory shad with a shad mouth of the Bay of Fundy (Huntsman doubts or with the alewives, which it resembles in the this record), and it usually is so uncommon within position of its dorsal fin, in the great depth of its our limits that we have seen none in the Gulf body, in its saw-toothed belly and in the lack of ourselves. -

South Western Nova Scotia
Netukulimk of Aquatic Natural Life “The N.C.N.S. Netukulimkewe’l Commission is the Natural Life Management Authority for the Large Community of Mi’kmaq /Aboriginal Peoples who continue to reside on Traditional Mi’Kmaq Territory in Nova Scotia undisplaced to Indian Act Reserves” P.O. Box 1320, Truro, N.S., B2N 5N2 Tel: 902-895-7050 Toll Free: 1-877-565-1752 2 Netukulimk of Aquatic Natural Life N.C.N.S. Netukulimkewe’l Commission Table of Contents: Page(s) The 1986 Proclamation by our late Mi’kmaq Grand Chief 4 The 1994 Commendation to all A.T.R.A. Netukli’tite’wk (Harvesters) 5 A Message From the N.C.N.S. Netukulimkewe’l Commission 6 Our Collective Rights Proclamation 7 A.T.R.A. Netukli’tite’wk (Harvester) Duties and Responsibilities 8-12 SCHEDULE I Responsible Netukulimkewe’l (Harvesting) Methods and Equipment 16 Dangers of Illegal Harvesting- Enjoy Safe Shellfish 17-19 Anglers Guide to Fishes Of Nova Scotia 20-21 SCHEDULE II Specific Species Exceptions 22 Mntmu’k, Saqskale’s, E’s and Nkata’laq (Oysters, Scallops, Clams and Mussels) 22 Maqtewe’kji’ka’w (Small Mouth Black Bass) 23 Elapaqnte’mat Ji’ka’w (Striped Bass) 24 Atoqwa’su (Trout), all types 25 Landlocked Plamu (Landlocked Salmon) 26 WenjiWape’k Mime’j (Atlantic Whitefish) 26 Lake Whitefish 26 Jakej (Lobster) 27 Other Species 33 Atlantic Plamu (Salmon) 34 Atlantic Plamu (Salmon) Netukulimk (Harvest) Zones, Seasons and Recommended Netukulimk (Harvest) Amounts: 55 SCHEDULE III Winter Lake Netukulimkewe’l (Harvesting) 56-62 Fishing and Water Safety 63 Protecting Our Community’s Aboriginal and Treaty Rights-Community 66-70 Dispositions and Appeals Regional Netukulimkewe’l Advisory Councils (R.N.A.C.’s) 74-75 Description of the 2018 N.C.N.S. -

A Report on Recapture from a 1968 Native Salmon Smolt Tagging
RESTRICTED THE NORTHWEST ATLANTIC FISHERIES INTERNA nONAl COMMISSION FOR ICES/ICNAF Salmon Doc. 71/15 Serial No. 2541 (also ICNAF Res.Doc. 71/71) (B.g.14) ANNUAL MEETING - JUNE 1971 A Report on Recapture from a 1968 Native Salmon Smolt Tagging Project on the Miramichi River by G.E. Turner Resource Development Branch Department of Fisheries and Forestry of Canada Halifax, Nova Scotis l.t INTRODUCTION A program to evaluate the contribution of hatchery reared salmon smolts to sport and commercial fisheries in the Atlantic region was started in 196B. Part of this program included the tagging of wild native smolts in selected.rivers for comparison of returns with hatchery releases. This report presents results on 1969 and 1970 recaptures of wild native smolts tagged and released in the Miramichi River in 196B. The Miramichi River (Figure 1) is located in New Brunswick, has a drainage area of over 5,500 square miles and empties into the Gulf of St. Lawrence. 2.0 METHODS AND MATERIALS The native smolts used for the tagging project were captured in the estuary on their seaward migration and are representativ~ of the whole river and not one particular tributary. Fish were selected for tagging because of their vigor and good physical appearance (lack of scaled areas, abrasions, fun gal growth or'decayed fins). Although size was not a determining factor in selection of smolts for tagging, those under 12 cm. were culled as a precaution against damage from tagging needles. Tags used on all smolts were the modified Carlin type tied with black monofilament nylon twine and applied with a double needle tagging jig. -

Fundy Routes
Fundy Region MAP ....................................................................................................Truro 1. La Plan che Rive r 2. Stew iack e Rive r 3. Rive r Heb 7 Route: No. 1 La Planche River Type: River Rating: easy Length: 30 kilometers round trip (18.5 miles) 2 days Portages: None Main bodies of water: La Planche River, Long Lake and Round Lake. Start: on the north side of the town of Amherst. Intermediate access: None Finish: Return by same route. This trip takes you up through a portion of the Tantramar Marshes. There is no white water and the current is not strong. There are no land marks that will be of any help and a number of side streams and ditches will make some navigational experience useful. The lower end of the river is tidal and the start should be made at high tide. The water levels are good except in extremely dry periods. On the north side of Long Lake you will pass the old abandoned ship railway that was built in the 1800’s to transport ships overland to the Northumberland Strait. The history of this can be found at Fort Beausejour on route 2 near Amherst. Fishing is good in certain areas and duck and muskrats are plentiful. There are not many good areas to camp along the river; but there are some good sites along the northeast shore of Long Lake. Detailed information: National Topographic Series Map No. 21H / 16E 8 Route: No. 2 Stewiacke River Type: River Rating: Moderate Length: 46 kilometers (28.7 miles) 2 days Portages: None Main bodies of water: Stewiacke River Start: Upper Stewiacke Intermediate access: at five locations. -

1 Atlantic Immigration Pilot Designated Employer List: The
Atlantic Immigration Pilot Designated Employer List: The following is a list of employers designated in New Brunswick through the Atlantic Immigration Pilot. This list does not indicate that these employers are hiring. To find current job vacancies got to www.nbjobs.ca. Liste des employeurs désignés Voici la liste des employeurs désignés sous le Projet pilote en matière d’immigration au Canada atlantique. Cette liste ne signifie pas que ces employeurs recrutent présentement.ss Pour les offres d’emploi, visitez le www.emploisnb.ca. Employer Name 3D Property Management 670807 NB Inc (Dépaneur Needs Caraquet & Shippagan) 693666 NB Inc. A & J Hanna Construction Ltd (Fredericton) A&W Miramichi (630883 NB Inc) A.C. Sharkey's Pub & Grill (Florenceville-Bristol) A.N.D. Communications A.R.Rietzel Landscaping Ltd Acadia Pizza Donair / Korean Restaurant (Dieppe) Acadia Veterinary Hospital Accor Hotels Global Reservation Centre Acorn Restaurant / Mads Truckstop (Lake George) Admiral's Quay B&B (Yang Developments Ltd.) Adorable Chocolat Inc Adrice Cormier Ltd Agence Résidentielle Restigouche Airport General Store (649459 NB Ltd) Airport Inn AirVM Albert's Draperies Alexandru & Camelia Trucking All Needs Special Care Inc. Allen, Paquet & Arseneau Allen's Petro Canada & Grocery (Allen's Enterprise Inc.) AL-Pack Amsterdam Inn & Suites Sussex (deWinter Brothers Ltd.) Andrei Chartovich 1 Employer Name Andrei Master Tailors Ltd Apex Industries Inc Appcast Armour Transport Inc Arom Chinese Cuisine Fredericton (655749 N.B. Ltd.) Asian Garden Indian Restaurant Moncton (Bhatia Brothers Ltd) Aspen University Association Multiculturelle du Restigouche Assurion Canada Inc Asurion Atelier Gérard Beaulieu Atlantic Ballet of Canada Atlantic Controls (Division of Laurentide Controls) Atlantic Home Improvement (656637 NB Inc) Atlantic Lottery Corporation Atlantic Pacific Transport Ltd.