Striped Bass Morone Saxatilis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Barriers to Fish Passage in Nova Scotia the Evolution of Water Control Barriers in Nova Scotia’S Watershed
Dalhousie University- Environmental Science Barriers to Fish Passage in Nova Scotia The Evolution of Water Control Barriers in Nova Scotia’s Watershed By: Gillian Fielding Supervisor: Shannon Sterling Submitted for ENVS 4901- Environmental Science Honours Abstract Loss of connectivity throughout river systems is one of the most serious effects dams impose on migrating fish species. I examine the extent and dates of aquatic habitat loss due to dam construction in two key salmon regions in Nova Scotia: Inner Bay of Fundy (IBoF) and the Southern Uplands (SU). This work is possible due to the recent progress in the water control structure inventory for the province of Nova Scotia (NSWCD) by Nova Scotia Environment. Findings indicate that 586 dams have been documented in the NSWCD inventory for the entire province. The most common main purpose of dams built throughout Nova Scotia is for hydropower production (21%) and only 14% of dams in the database contain associated fish passage technology. Findings indicate that the SU is impacted by 279 dams, resulting in an upstream habitat loss of 3,008 km of stream length, equivalent to 9.28% of the total stream length within the SU. The most extensive amount of loss occurred from 1920-1930. The IBoF was found to have 131 dams resulting in an upstream habitat loss of 1, 299 km of stream length, equivalent to 7.1% of total stream length. The most extensive amount of upstream habitat loss occurred from 1930-1940. I also examined if given what I have learned about the locations and dates of dam installations, are existent fish population data sufficient to assess the impacts of dams on the IBoF and SU Atlantic salmon populations in Nova Scotia? Results indicate that dams have caused a widespread upstream loss of freshwater habitat in Nova Scotia howeverfish population data do not exist to examine the direct impact of dam construction on the IBoF and SU Atlantic salmon populations in Nova Scotia. -

Striped Bass Conservation Measures Needed in New
WAIITIENTof the INTERIOR newsrelease FISH AND WILDLIFE SERVICE NATIONAL MARINE FISHERIES SERVICE For Release February 10, 1987 FWS--Megan Durham 202-343-5634 NMFS--Brian Gorman 202-673-5445 STRIPED BASS CONSERVATION MEASURESNEEDED IN NEW JERSEY, DISTRICT Ok COLUUBIA TO Am)10 I-tDtRAL FIm BAN The Departments of the Interior and Commerce will publish joint notices in the February 12 Federal Register announcing that they intend to impose a moratorium on striped bass fishing in the waters of the District of Columbia and coastal waters of New Jersey because the District and New Jersey are not in compliance with the Atlantic Striped Bass Conservation Act. Letters announcing the decision have been sent to New Jersey Governor Thomas H. Kean and District Mayor Marion Barry, signed by William Horn, Assistant Secretary of the Interior for Fish and Wildlife and Parks, and Anthony J. Calio, Under Secretary of Commerce for Oceans and Atmosphere. If New Jersey and the District do not adopt regulations to protect female striped bass from the Chesapeake Bay, Interior and Commerce will impose the striped bass fishing moratorium effective April 1, the agencies said. The letter to Mayor Barry commended the District's progress in developing regulations and said that, based on available information, it appears that the regulations being developed would, if adopted, bring the District into compliance and eliminate the need for a fishing moratorium. The letter to Governor Kean encouraged New Jersey to take the resource conservation measures necessary to meet the requirements of the Atlantic Striped Bass Conservation Act. The Act applies to coastal migratory stocks of striped bass occurring from Maine to North Carolina. -
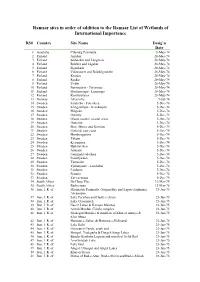
Ramsar Sites in Order of Addition to the Ramsar List of Wetlands of International Importance
Ramsar sites in order of addition to the Ramsar List of Wetlands of International Importance RS# Country Site Name Desig’n Date 1 Australia Cobourg Peninsula 8-May-74 2 Finland Aspskär 28-May-74 3 Finland Söderskär and Långören 28-May-74 4 Finland Björkör and Lågskär 28-May-74 5 Finland Signilskär 28-May-74 6 Finland Valassaaret and Björkögrunden 28-May-74 7 Finland Krunnit 28-May-74 8 Finland Ruskis 28-May-74 9 Finland Viikki 28-May-74 10 Finland Suomujärvi - Patvinsuo 28-May-74 11 Finland Martimoaapa - Lumiaapa 28-May-74 12 Finland Koitilaiskaira 28-May-74 13 Norway Åkersvika 9-Jul-74 14 Sweden Falsterbo - Foteviken 5-Dec-74 15 Sweden Klingavälsån - Krankesjön 5-Dec-74 16 Sweden Helgeån 5-Dec-74 17 Sweden Ottenby 5-Dec-74 18 Sweden Öland, eastern coastal areas 5-Dec-74 19 Sweden Getterön 5-Dec-74 20 Sweden Store Mosse and Kävsjön 5-Dec-74 21 Sweden Gotland, east coast 5-Dec-74 22 Sweden Hornborgasjön 5-Dec-74 23 Sweden Tåkern 5-Dec-74 24 Sweden Kvismaren 5-Dec-74 25 Sweden Hjälstaviken 5-Dec-74 26 Sweden Ånnsjön 5-Dec-74 27 Sweden Gammelstadsviken 5-Dec-74 28 Sweden Persöfjärden 5-Dec-74 29 Sweden Tärnasjön 5-Dec-74 30 Sweden Tjålmejaure - Laisdalen 5-Dec-74 31 Sweden Laidaure 5-Dec-74 32 Sweden Sjaunja 5-Dec-74 33 Sweden Tavvavuoma 5-Dec-74 34 South Africa De Hoop Vlei 12-Mar-75 35 South Africa Barberspan 12-Mar-75 36 Iran, I. R. -

Flood Frequency Analyses for New Brunswick Rivers Canadian Technical Report of Fisheries and Aquatic Sciences 2920
Flood Frequency Analyses for New Brunswick Rivers Aucoin, F., D. Caissie, N. El-Jabi and N. Turkkan Department of Fisheries and Oceans Gulf Region Oceans and Science Branch Diadromous Fish Section P.O. Box 5030, Moncton, NB, E1C 9B6 2011 Canadian Technical Report of Fisheries and Aquatic Sciences 2920 Canadian Technical Report of Fisheries and Aquatic Sciences Technical reports contain scientific and technical information that contributes to existing knowledge but which is not normally appropriate for primary literature. Technical reports are directed primarily toward a worldwide audience and have an international distribution. No restriction is placed on subject matter and the series reflects the broad interests and policies of Fisheries and Oceans, namely, fisheries and aquatic sciences. Technical reports may be cited as full publications. The correct citation appears above the abstract of each report. Each report is abstracted in the data base Aquatic Sciences and Fisheries Abstracts. Technical reports are produced regionally but are numbered nationally. Requests for individual reports will be filled by the issuing establishment listed on the front cover and title page. Numbers 1-456 in this series were issued as Technical Reports of the Fisheries Research Board of Canada. Numbers 457-714 were issued as Department of the Environment, Fisheries and Marine Service, Research and Development Directorate Technical Reports. Numbers 715-924 were issued as Department of Fisheries and Environment, Fisheries and Marine Service Technical Reports. The current series name was changed with report number 925. Rapport technique canadien des sciences halieutiques et aquatiques Les rapports techniques contiennent des renseignements scientifiques et techniques qui constituent une contribution aux connaissances actuelles, mais qui ne sont pas normalement appropriés pour la publication dans un journal scientifique. -

A Review of Ice and Tide Observations in the Bay of Fundy
A tlantic Geology 195 A review of ice and tide observations in the Bay of Fundy ConDesplanque1 and David J. Mossman2 127 Harding Avenue, Amherst, Nova Scotia B4H 2A8, Canada departm ent of Physics, Engineering and Geoscience, Mount Allison University, 67 York Street, Sackville, New Brunswick E4L 1E6, Canada Date Received April 27, 1998 Date Accepted December 15,1998 Vigorous quasi-equilibrium conditions characterize interactions between land and sea in macrotidal regions. Ephemeral on the scale of geologic time, estuaries around the Bay of Fundy progressively infill with sediments as eustatic sea level rises, forcing fringing salt marshes to form and reform at successively higher levels. Although closely linked to a regime of tides with large amplitude and strong tidal currents, salt marshes near the Bay of Fundy rarely experience overflow. Built up to a level about 1.2 m lower than the highest astronomical tide, only very large tides are able to cover the marshes with a significant depth of water. Peak tides arrive in sets at periods of 7 months, 4.53 years and 18.03 years. Consequently, for months on end, no tidal flooding of the marshes occurs. Most salt marshes are raised to the level of the average tide of the 18-year cycle. The number of tides that can exceed a certain elevation in any given year depends on whether the three main tide-generating factors peak at the same time. Marigrams constructed for the Shubenacadie and Cornwallis river estuaries, Nova Scotia, illustrate how the estuarine tidal wave is reshaped over its course, to form bores, and varies in its sediment-carrying and erosional capacity as a result of changing water-surface gradients. -

Tennessee Fish Species
The Angler’s Guide To TennesseeIncluding Aquatic Nuisance SpeciesFish Published by the Tennessee Wildlife Resources Agency Cover photograph Paul Shaw Graphics Designer Raleigh Holtam Thanks to the TWRA Fisheries Staff for their review and contributions to this publication. Special thanks to those that provided pictures for use in this publication. Partial funding of this publication was provided by a grant from the United States Fish & Wildlife Service through the Aquatic Nuisance Species Task Force. Tennessee Wildlife Resources Agency Authorization No. 328898, 58,500 copies, January, 2012. This public document was promulgated at a cost of $.42 per copy. Equal opportunity to participate in and benefit from programs of the Tennessee Wildlife Resources Agency is available to all persons without regard to their race, color, national origin, sex, age, dis- ability, or military service. TWRA is also an equal opportunity/equal access employer. Questions should be directed to TWRA, Human Resources Office, P.O. Box 40747, Nashville, TN 37204, (615) 781-6594 (TDD 781-6691), or to the U.S. Fish and Wildlife Service, Office for Human Resources, 4401 N. Fairfax Dr., Arlington, VA 22203. Contents Introduction ...............................................................................1 About Fish ..................................................................................2 Black Bass ...................................................................................3 Crappie ........................................................................................7 -

EBFM Covers:EBFM-Striped Bass Cover-8.5X11
ECOSYSTEM BASED FISHERIES MANAGEMENT FOR CHESAPEAKE BAY Menhaden Species Team Background and Issue Briefs The Ecosystem-Based Fisheries Management (EBFM) Project for Chesapeake Bay has been developed and coordinated by Maryland Sea Grant, working in partnership with the scientific community and the region’s state and federal agencies (the Virginia Marine Resources Commission, Maryland Department of Natural Resources, Potomac River Fisheries Commission, Atlantic States Marine Fisheries Commission, District of Columbia Department of the Environment, NOAA, and EPA). The EBFM Project targets five key species identified in the Ecosystem Planning for Chesapeake Bay document, including striped bass, menhaden, blue crab, alosines, and oysters. The goals of the EBFM project are to build a sustainable mechanism for addressing ecosystem issues for fisheries within Chesapeake Bay and to develop ecosystem tools for use in ecosystem-based fishery management plans for the five key species (or group of species in the case of alosines). Currently the project involves 85 scientists, managers, and stakeholders from within and beyond the Chesapeake Bay region. For more information on Maryland Sea Grant’s Ecosystem-Based Fishery Management Project please visit: www.mdsg.umd.edu/ebfm. This publication was produced by Maryland Sea Grant. Ecosystem-Based Fisheries Management for Chesapeake Bay: Menhaden Background and Issue Briefs. 2009. EBFM Menhaden Species Team. Publication Number UM-SG-TS-2009-08 Maryland Sea Grant 4321 Hartwick Rd., Suite 300 College Park, -

Final Biological Assessment for Roseate Tern, New
FINAL BIOLOGICAL ASSESSMENT for the ROSEATE TERN NEW BEDFORD HARBOR – SOUTH TERMINAL PROJECT NEW BEDFORD, MASSACHUSETTS U.S. Environmental Protection Agency Office of Ecosystem Protection (OEP05-2)) U.S. EPA New England Region 5 Post Office Square, Suite 100 Boston, MA 02109-3912 July 2012 TABLE OF CONTENTS Subject Page I. Introduction 3 II. Description of Project and Action Area 4 A. Project Description 4 B. Action Area 6 III. Environmental Setting 6 A. Flora - Salt Marsh, Intertidal and Subtidal Resources 6 B. Fauna – Finfish and Shellfish 7 C. Physical Conditions – Sediments, Patterns of Circulation, Noise 11 IV. Roseate Tern Biology 12 A. Seasonal Distribution 12 B. Nesting 12 C. Staging 14 D. Foraging 14 V. Effects Analysis 17 A. Direct Loss of Salt Marsh, Intertidal and Subtidal habitat 17 B. Foraging by Nesting/Migrating Terns 18 C. Effects on Prey Species In Shallow Water Habitat 18 D. Dredging Impacts to Prey Fish in Sub-tidal Environment 19 E. Noise and Traffic 20 F. Oil Spills and Shipping Traffic 21 G. Ecological Benefits of the Project 22 VI. Determination of Effects on the Roseate Tern 22 VII Conclusion 23 VIII. References. 24 IX. List of Contacts Made and Preparers 28 2 New Bedford Harbor - South Terminal Project Endangered Species Act Biological Assessment for the Roseate Tern I. Introduction This Biological Assessment (BA) was prepared to comply with Section 7 of the Endangered Species Act (ESA). It assesses the potential effects of the construction and long-term operation of the proposed New Bedford Harbor (NBH) - South Terminal project in New Bedford, MA, on the roseate tern (Sterna dougallii), a federally listed as endangered which may occur in the area of the proposed project.1 While New Bedford Harbor is not federally designated critical habitat for any federally endangered species, the project area provides potential habitat for nesting and foraging for the roseate tern. -

Status and Conservation of Eelgrass (Zostera Marina) in Eastern Canada
Status and Conservation of Eelgrass (Zostera marina) in Eastern Canada Alan R. Hanson Atlantic Region 2004 Canadian Wildlife Service Environmental Conservation Branch Technical Report Series Number 412 TECHNICAL REPORT SERIES CANADIAN WILDLIFE SERVICE This series of reports, established in 1986, contains technical and scientific information from projects of the Canadian Wildlife Service. The reports are intended to make available material that either is of interest to a limited audience or is too extensive to be accommodated in scientific journals or in existing CWS series. Demand for these Technical Reports is usually confined to specialists in the fields concerned. Consequently, they are produced regionally and in small quantities; they can be obtained only from the address given on the title page. However, they are numbered nationally. The recommended citation appears on the title page. Technical Reports are available in CWS libraries and are listed in the catalogue of the National Library of Canada in scientific libraries across Canada. They are printed in the official language chosen by the author to meet the language preference of the likely audience, with a résumé in the second official language. To determine whether there is significant demand for making the reports available in the second official language, CWS invites users to specify their official language preference. Requests for Technical Reports in the second official language should be sent to the address on the title page. SÉRIE DE RAPPORTS TECHNIQUES DU SERVICE CANADIEN DE LA FAUNE Cette série de rapports donnant des informations scientifiques et techniques sur les projets du Service canadien de la faune (SCF) a démarré en 1986. -

C S a S S C É S Canadian Stock Assessment Secretariat Secrétariat Canadien Pour L’Évaluation Des Stocks
Fisheries and Oceans Pêches et Océans Science Sciences C S A S S C É S Canadian Stock Assessment Secretariat Secrétariat canadien pour l’évaluation des stocks Document de recherche 2000/007 Research Document 2000/007 Not to be cited without Ne pas citer sans permission of the authors 1 autorisation des auteurs 1 Atlantic salmon (Salmo salar L.) stock status on rivers in the Northumberland Strait, Nova Scotia area, in 1999 S. F. O’Neil, K.A. Rutherford, and D. Aitken Diadromous Fish Division Science Branch, Maritimes Region Bedford Institute of Oceanography P.O.Box 1006 Dartmouth, N.S. B2Y 4A2 1 This series documents the scientific basis for 1 La présente série documente les bases the evaluation of fisheries resources in scientifiques des évaluations des ressources Canada. As such, it addresses the issues of halieutiques du Canada. Elle traite des the day in the time frames required and the problèmes courants selon les échéanciers documents it contains are not intended as dictés. Les documents qu’elle contient ne definitive statements on the subjects doivent pas être considérés comme des addressed but rather as progress reports on énoncés définitifs sur les sujets traités, mais ongoing investigations. plutôt comme des rapports d’étape sur les études en cours. Research documents are produced in the Les documents de recherche sont publiés dans official language in which they are provided to la langue officielle utilisée dans le manuscrit the Secretariat. envoyé au Secrétariat. This document is available on the Internet at: Ce document est disponible sur l’Internet à: http://www.dfo-mpo.gc.ca/csas/ ISSN 1480-4883 Ottawa, 2000 i Abstract Fifteen separate rivers on the Northumberland Strait shore of Nova Scotia support Atlantic salmon stocks. -

Atlantic Salmon Stock Status on Rivers in the Northumberland Strait, Nova
Department of Fisheries and Oceans Ministère des pêches et océan s Canadian Stock Assessment Secretariat Secrétariat canadien pour l'évaluation des -stocks Research Document 97/22 Document de recherche 97/22 Not to be cited without Ne pas citer sans permission of the authors ' autorisation des auteurs ' Atlantic salmon (Salmo salar L .) stock status on rivers in the Northumberland Strait, Nova Scotia area, in 1996 S. F. O'Neil, D. A. Longard, and C . J. Harvie Diadromous Fish Division Science Branch Maritimes Region P.O.Box 550 Halifax, N .S. B3J 2S7 ' This series documents the scientific basis for ' La présente série documente les bases the evaluation of fisheries resources in Canada . scientifiques des évaluations des ressources As such, it addresses the issues of the day in halieutiques du Canada. Elle traite des the time frames required and the documents it problèmes courants selon les échéanciers contains are not intended as definitive dictés. Les documents qu'elle contient ne statements on the subjects addressed but doivent pas être considérés comme des rather as progress reports on ongoing énoncés définitifs sur les sujets traités, mais investigations . plutôt comme des rapports d'étape sur les études en cours. Research documents are produced in the Les documents de recherche sont publiés dans official language in which they are provided to la langue officielle utilisée dans le manuscrit the Secretariat . envoyé au secrétariat . ii Abstrac t Fifteen separate rivers on the Northumberland Strait shore of Nova Scotia support Atlantic salmon stocks. Stock status information for 1996 is provided for nine of those stocks based on the conservation requirements and escapements calculated either from mark-and-recapture experiments (East River, Pictou and River Philip) or capture (exploitation) rates in the angling fishery. -

2021 Striped Bass Regulations
2021 MAINE STRIPED BASS REGULATIONS If you are a recreational saltwater fisherman, Maine law may require you to register with the Maine Saltwater Recreational Fishing Registry. To learn more or to register visit: www.maine.gov/saltwater or call 207-633-9500. The following Maine saltwater recreational fishing regulations are current as of June 8, 2021. However, they are subject to change. Please contact our office or your local Marine Patrol Officer with questions. All minimum lengths are total length, NOT fork length. The sale of fish by recreational anglers is prohibited. Maine’s striped bass regulations cover all Maine coastal waters up to the head of tide in all rivers. In addition, there are special regulations in effect from December 1 through June 30 in the Kennebec, Sheepscot and Androscoggin Rivers and all related tributaries (see “SPECIAL KENNEBEC REGULATIONS” below). FEDERAL REGULATION It is unlawful to fish for, take or possess striped bass in Federal waters (waters greater than 3 miles from shore). STATEWIDE REGULATIONS OPEN SEASON January 1 through December 31, inclusive (except the Kennebec watershed, see below). BAG LIMITS A person may take and possess 1 fish per day. SIZE LIMITS The fish must be equal to or greater than 28 inches and less than 35 inches total length. “TOTAL LENGTH” is a straight line measurement from the lower jaw to the tip of the tail with the tail pinched together. DISPOSITION Personal use only, sale is prohibited. Fish must remain whole and intact. GENERAL GEAR RESTRICTIONS • Hook and line only, no gaffing of striped bass. • No bait allowed when using treble hooks.