Tracheary Element Differentiation and Secondary Cell-Wall Formation in Cell Cultures of Coniferous Gymnosperms*
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Non-Wood Forest Products from Conifers
Page 1 of 8 NON -WOOD FOREST PRODUCTS 12 Non-Wood Forest Products From Conifers FAO - Food and Agriculture Organization of the United Nations The designations employed and the presentation of material in this publication do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. M-37 ISBN 92-5-104212-8 (c) FAO 1995 TABLE OF CONTENTS FOREWORD ACKNOWLEDGMENTS ABBREVIATIONS INTRODUCTION CHAPTER 1 - AN OVERVIEW OF THE CONIFERS WHAT ARE CONIFERS? DISTRIBUTION AND ABUNDANCE USES CHAPTER 2 - CONIFERS IN HUMAN CULTURE FOLKLORE AND MYTHOLOGY RELIGION POLITICAL SYMBOLS ART CHAPTER 3 - WHOLE TREES LANDSCAPE AND ORNAMENTAL TREES Page 2 of 8 Historical aspects Benefits Species Uses Foliage effect Specimen and character trees Shelter, screening and backcloth plantings Hedges CHRISTMAS TREES Historical aspects Species Abies spp Picea spp Pinus spp Pseudotsuga menziesii Other species Production and trade BONSAI Historical aspects Bonsai as an art form Bonsai cultivation Species Current status TOPIARY CONIFERS AS HOUSE PLANTS CHAPTER 4 - FOLIAGE EVERGREEN BOUGHS Uses Species Harvesting, management and trade PINE NEEDLES Mulch Decorative baskets OTHER USES OF CONIFER FOLIAGE CHAPTER 5 - BARK AND ROOTS TRADITIONAL USES Inner bark as food Medicinal uses Natural dyes Other uses TAXOL Description and uses Harvesting methods Alternative -

Phytosociological Analysis of Pine Forest at Indus Kohistan, Kpk, Pakistan
Pak. J. Bot., 48(2): 575-580, 2016. PHYTOSOCIOLOGICAL ANALYSIS OF PINE FOREST AT INDUS KOHISTAN, KPK, PAKISTAN ADAM KHAN1, MOINUDDIN AHMED2, MUHAMMAD FAHEEM SIDDIQUI*3, JAVED IQBAL1 AND MUHAMMAD WAHAB4 1Laboratory of plant ecology and Dendrochronology, Department of Botany, Federal Urdu University, Gulshan-e-Iqbal Campus Karachi, Pakistan 2Department of Earth and Environmental Systems, 600 Chestnut Street Indiana State University, Terre Haute, IN, USA 3Department of Botany, University of Karachi, Karachi-75270, Pakistan 4Institute of Botany, Chinese Academy of Sciences, Beijing, China *Corresponding author’s email: [email protected] Abstract The study was carried out to describe the pine communities at Indus Kohistan valley in quantitative term. Thirty stands of relatively undisturbed vegetation were selected for sampling. Quantitative sampling was carried out by Point Centered Quarter (PCQ) method. Seven tree species were common in the Indus Kohistan valley. Cedrus deodara was recorded from twenty eight different locations and exhibited the highest mean importance value while Pinus wallichiana was recorded from 23 different locations and exhibited second highest mean importance value. Third most occurring species was Abies pindrow that attained the third highest mean importance value and Picea smithiana was recorded from eight different locations and attained fourth highest importance value while it was first dominant in one stand and second dominant in four stands. Pinus gerardiana, Quercus baloot and Taxus fuana were the rare species in this area, these species attained low mean importance value. Six communities and four monospecific stands of Cedrus deodara were recognized. Cedrus-Pinus community was the most occurring community, which was recorded from 13 different stands. -
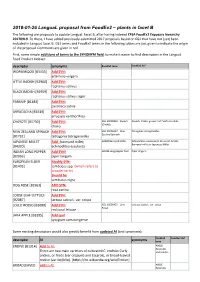
2018-01-26 Langual Proposal from Foodex2 – Plants in Facet B
2018-01-26 LanguaL proposal from FoodEx2 – plants in facet B The following are proposals to update LanguaL Facet B, after having indexed EFSA FoodEx2 Exposure hierarchy 20170919. To these, I have added previously-submitted 2017 proposals based on GS1 that have not (yet) been included in LanguaL facet B. GS1 terms and FoodEx2 terms in the following tables are just given to indicate the origin of the proposal. Comments are given in red. First, some simple additions of terms to the SYNONYM field, to make it easier to find descriptors in the LanguaL Food Product Indexer: descriptor synonyms FoodEx2 term FoodEx2 def WORMWOOD [B3433] Add SYN: artemisia vulgaris LITTLE RADISH [B2960] Add SYN: raphanus sativus BLACK RADISH [B2959] Add SYN: raphanus sativus niger PARSNIP [B1483] Add SYN: pastinaca sativa ARRACACHA [B3439] Add SYN: arracacia xanthorrhiza CHAYOTE [B1730] Add SYN: GS1 10006356 - Squash Squash, Choko, grown from Sechium edule (Choko) choko NEW ZEALAND SPINACH Add SYN: GS1 10006427 - New- Tetragonia tetragonoides Zealand Spinach [B1732] tetragonia tetragonoides JAPANESE MILLET Add : barnyard millet; A000Z Barnyard millet Echinochloa esculenta (A. Braun) H. Scholz, Barnyard millet or Japanese Millet. [B4320] echinochloa esculenta INDIAN LONG PEPPER Add SYN! A019B Long pepper fruit Piper longum [B2956] piper longum EUROPEAN ELDER Modify SYN: [B1403] sambucus spp. (which refers to broader term) Should be sambucus nigra DOG ROSE [B2961] ADD SYN: rosa canina LOOSE LEAF LETTUCE Add SYN: [B2087] lactusa sativa L. var. crispa LOLLO ROSSO [B2088] Add SYN: GS1 10006425 - Lollo Lactuca sativa L. var. crispa Rosso red coral lettuce JAVA APPLE [B3395] Add syn! syzygium samarangense Some existing descriptors would also greatly benefit from updated AI (and synonyms): FoodEx2 FoodEx2 def descriptor AI synonyms term ENDIVE [B1314] Add to AI: A00LD Escaroles There are two main varieties of cultivated C. -

Biodiversity Conservation in Botanical Gardens
AgroSMART 2019 International scientific and practical conference ``AgroSMART - Smart solutions for agriculture'' Volume 2019 Conference Paper Biodiversity Conservation in Botanical Gardens: The Collection of Pinaceae Representatives in the Greenhouses of Peter the Great Botanical Garden (BIN RAN) E M Arnautova and M A Yaroslavceva Department of Botanical garden, BIN RAN, Saint-Petersburg, Russia Abstract The work researches the role of botanical gardens in biodiversity conservation. It cites the total number of rare and endangered plants in the greenhouse collection of Peter the Great Botanical garden (BIN RAN). The greenhouse collection of Pinaceae representatives has been analysed, provided with a short description of family, genus and certain species, presented in the collection. The article highlights the importance of Pinaceae for various industries, decorative value of plants of this group, the worth of the pinaceous as having environment-improving properties. In Corresponding Author: the greenhouses there are 37 species of Pinaceae, of 7 geni, all species have a E M Arnautova conservation status: CR -- 2 species, EN -- 3 species, VU- 3 species, NT -- 4 species, LC [email protected] -- 25 species. For most species it is indicated what causes depletion. Most often it is Received: 25 October 2019 the destruction of natural habitats, uncontrolled clearance, insect invasion and diseases. Accepted: 15 November 2019 Published: 25 November 2019 Keywords: biodiversity, botanical gardens, collections of tropical and subtropical plants, Pinaceae plants, conservation status Publishing services provided by Knowledge E E M Arnautova and M A Yaroslavceva. This article is distributed under the terms of the Creative Commons 1. Introduction Attribution License, which permits unrestricted use and Nowadays research of biodiversity is believed to be one of the overarching goals for redistribution provided that the original author and source are the modern world. -

Disturbances Influence Trait Evolution in Pinus
Master's Thesis Diversify or specialize: Disturbances influence trait evolution in Pinus Supervision by: Prof. Dr. Elena Conti & Dr. Niklaus E. Zimmermann University of Zurich, Institute of Systematic Botany & Swiss Federal Research Institute WSL Birmensdorf Landscape Dynamics Bianca Saladin October 2013 Front page: Forest of Pinus taeda, northern Florida, 1/2013 Table of content 1 STRONG PHYLOGENETIC SIGNAL IN PINE TRAITS 5 1.1 ABSTRACT 5 1.2 INTRODUCTION 5 1.3 MATERIAL AND METHODS 8 1.3.1 PHYLOGENETIC INFERENCE 8 1.3.2 TRAIT DATA 9 1.3.3 PHYLOGENETIC SIGNAL 9 1.4 RESULTS 11 1.4.1 PHYLOGENETIC INFERENCE 11 1.4.2 PHYLOGENETIC SIGNAL 12 1.5 DISCUSSION 14 1.5.1 PHYLOGENETIC INFERENCE 14 1.5.2 PHYLOGENETIC SIGNAL 16 1.6 CONCLUSION 17 1.7 ACKNOWLEDGEMENTS 17 1.8 REFERENCES 19 2 THE ROLE OF FIRE IN TRIGGERING DIVERSIFICATION RATES IN PINE SPECIES 21 2.1 ABSTRACT 21 2.2 INTRODUCTION 21 2.3 MATERIAL AND METHODS 24 2.3.1 PHYLOGENETIC INFERENCE 24 2.3.2 DIVERSIFICATION RATE 24 2.4 RESULTS 25 2.4.1 PHYLOGENETIC INFERENCE 25 2.4.2 DIVERSIFICATION RATE 25 2.5 DISCUSSION 29 2.5.1 DIVERSIFICATION RATE IN RESPONSE TO FIRE ADAPTATIONS 29 2.5.2 DIVERSIFICATION RATE IN RESPONSE TO DISTURBANCE, STRESS AND PLEIOTROPIC COSTS 30 2.5.3 CRITICAL EVALUATION OF THE ANALYSIS PATHWAY 33 2.5.4 PHYLOGENETIC INFERENCE 34 2.6 CONCLUSIONS AND OUTLOOK 34 2.7 ACKNOWLEDGEMENTS 35 2.8 REFERENCES 36 3 SUPPLEMENTARY MATERIAL 39 3.1 S1 - ACCESSION NUMBERS OF GENE SEQUENCES 40 3.2 S2 - TRAIT DATABASE 44 3.3 S3 - SPECIES DISTRIBUTION MAPS 58 3.4 S4 - DISTRIBUTION OF TRAITS OVER PHYLOGENY 81 3.5 S5 - PHYLOGENETIC SIGNAL OF 19 BIOCLIM VARIABLES 84 3.6 S6 – COMPLETE LIST OF REFERENCES 85 2 Introduction to the Master's thesis The aim of my master's thesis was to assess trait and niche evolution in pines within a phylogenetic comparative framework. -

Studies on Drying, Packaging and Storage of Solar Tunnel Dried Chilgoza Nuts
Available online a t www.scholarsresearchlibrary.com Scholars Research Library Archives of Applied Science Research, 2012, 4 (3):1311-1319 (http://scholarsresearchlibrary.com/archive.html) ISSN 0975-508X CODEN (USA) AASRC9 Studies on drying, packaging and storage of solar tunnel dried chilgoza nuts N. S Thakur, Sharma S, Joshi V. K, Thakur K. S and Jindal N Department of Food Science and Technology, Dr YS Parmar University of Horticulture and Forestry, Nauni-Solan, HP ______________________________________________________________________________ ABSTRACT Chilgoza (Pinus gerardiana) is one of the pine nuts among six species found in India which produce edible nuts. Because of the traditional handling of this nut by tribals, it lasts only for few weeks in the market. Studies were undertaken to compare the solar drying modes for drying of this nut and screen out the suitable packaging material for its storage. Extracted nuts were dried under three solar drying means like solar cabinet drier (46-52 ⁰C), solar tunnel drier (43-47 ⁰C) and open sun (18-22 ⁰C). Solar tunnel drier was found to be best drying mode for drying quality nuts as compare to the others. So, nuts dried in this drier were packed in five different packaging materials and stored under ambient conditions for six months. The some physico-chemical quality characteristics like a w (0.208), oil (49.1%) total carbohydrates ( 24.9%), and proteins ( 11.8%) and sensory quality attributes of packed nuts were retained better in glass jars closely followed by aluminum laminate pouch after six months of storage as compared to others. Solar tunnel drier was the best drying mode and glass jar as well as aluminum laminate pouch were the best materials for packaging and storage. -

Culture of Gymnosperm Tissue in Vitro
Culture of Gymnosperm tissue in vitro R.N. Konar and Chitrita Guha Department ofBotany, University ofDelhi, Delhi 7, India SUMMARY A review is given of recent advances in the culture of vegetative and repoductive tissues of Gymnosperms. Gymnosperm tissue culture is still in its infancy as compared to its angiosperm of efforts raise counterpart. In spite numerous to cultures from time to time little success has so far been achieved. 1. CULTURE OF VEGETATIVE PARTS Growth and development of callus Success in maintaining a continuous culture of coniferous tissue in vitro was first reported by Ball (1950). He raised tissues from the young adventive shoots burls of growing on the Sequoia sempervirens on diluted Knop’s solution with 3 per cent sucrose and 1 ppm 1AA. Marginal meristems, cambium-like meris- around of tracheids and cells could be tems groups mature parenchymatous distinguished in the callus mass. The parenchymatous cells occasionally con- tained tannin. the of tannin is According to Ball (1950) anatomically presence not inconsistent with the normal function of the shoot apex. He considered that probably the tannin cells have less potentialities to develop. Reinert & White (1956) cultured the normal and tumorous tissues of Picea glauca. They excised the cambial region from the tumorous (characteristic ofthe species) and non-tumorous portions of tumor bearing trees and also cambium from normal trees. This work was carried out with a view to understand the de- gree ofmalignancy of the cells and the biochemical characteristics of the tumors. They developed a rather complex nutrient medium consisting of White’s mine- rals, 16 amino acids and amides, 8 vitamins and auxin to raise the cultures. -

An Updated List of Species Used in Tree-Ring Research
TREE-RING BULLETIN, Vol. 53, 1993 AN UPDATED LIST OF SPECIES USED IN TREE-RING RESEARCH HENRI D. GRISSINO-MAYER Laboratory of Tree-Ring Research University of Arizona Tucson, AZ 85721, U.S.A. ABSTRACT During the past 100 years, researchers have investigated the potential of hundreds of tree and shrub species for use in applications of tree-ring research. Although several lists of species known to crossdate have been published, investigated species that do not crossdate are rarely included despite the usefulness of this infonnation for future research. This paper provides a list of the Latin and common names of 573 species that have been investigated in tree-ring research, infor mation on species known to crossdate, and information on species with measurement and/or chronology data in the International Tree-Ring Data Bank. In addition, a measure of the suitability of a species for future tree-ring applications, the Crossdating Index (CDI), is developed and pro posed for standard usage. 1n den letzten hundert J ahren haben Forscher das Potential von hunderten von Baum- und Buscharten fi.ir die Anwendung in der Jahresring-Forschung untersucht. Zahlreiche Listen mit Arten, von denen man wei~, da~ sie zeitlich korrespondieren, sind bereits veroffentlicht worden, dagegen sind untersuchte Arten, die nicht zeitlich korresponclieren, selten in Publikationen beriick sichtigt worden, obwohl diese Informationen fi.ir die kiinftige Forschung nutzvoll sein konnten. Dieser Artikel legt eine Liste der lateinischen und der gemeinen Narnen von 573 Arten vor, die im Rahmen der Jahresring-Forschung untersucht worden sind, Inforrnationen Uber Arten, die bekan nterweise zeitlich korrespondieren sowie Informationen iiber Arten mit Ma~- und/oder Chronologiedaten in der intemationalen Jahresring-Datenbank (International Tree-Ring Data Bank). -

Regeneration of Cold Desert Pine of N.W
Regeneration of Cold Desert Pine of N.W. Himalayas (India)—A Preliminary Study T. N. Lakhanpal Sunil Kumar Abstract—The cold desert pine of India, Pinus gerardiana (Wall.) fifth, heavy and unrestricted sheep and goat grazing causes has been subjected to overexploitation because of the commercial a lot of damage to young seedlings (Chauhan 1986). All value of its edible seeds and ethnic uses. Regeneration is deficient. these factors reduce the chances of natural regeneration Preliminary studies conducted by inoculating the seedlings with of this pine. Severe biotic interference and lack of regener- mycorrhiza show great promise in establishment and performance ation in this pine may result in the extinction of this species of the seedlings. (Kumar 1986; Sehgal and Chauhan 1989). For regeneration, it has been suggested that areas bearing chilgoza pine should be closed for a period of 30 years for rights holders. Artificial regeneration has been achieved at Pinus gerardiana, commonly and commercially known a number of places both by sowing and planting of nursery as ‘chilgoza’ and/or ‘neoza’ pine, is a forest tree restricted raised plants at Kalpa, Ralli (Kilba Range), Akpa (Morrand in India to dry inner valleys of the Northwest Himalayas Range), Shongtong, and Purbani (Kalpa Range) (Chauhan (1,600 to 3,000 m elevation). It occurs in Kinnaur (Satluj 1986). Valley) and Pangi in Himachal Pradesh (Ravi and Chenab However, no attention has ever been paid to the use of Valleys) extending westward to Kashmir, Afghanistan, mycorrhiza for artificial inoculation of chilgoza pine seed- and Northern Baluchistan. lings. Saplings are usually planted after they attain a Neoza pine grows gregariously, forming forests of a some- height of about 5 to 10 cm and are 3 to 4 years old. -

Non-Wood Forest Products from Conifers
NO\ -WOOD FOREST PROaCTS 12 Non-wood forest products from conifers Food and Agriculture Organizahon of the United Nations NO \--WOOD FOREST PRODUCTS 12 Non-wood forest products from conifers by William M. Ciesla European Forest Institute FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS Rome, 1998 Reprinted 2001 This paper discusses both traditional and contemporary uses of products from conifers. This material is presented for information only and does not imply endorsement by the author or by FAO. Some of those products have medicinal purposes; however, they should only be used under the care and guidance of a qualified physician. Transport of certain non-wood forest products (e.g. foliage, Christmas trees, seeds and landscape or ornamental plants) across international boundaries poses a risk of accidental transport and introduction of insects, fungi or other potentially destructive agents.Itis recommended that anyone planning to move plant materials across international boundaries check with appropriate authorities in the country from which the products are to be exported and the countries into which the products are to be imported for import permit requirements or restrictions which might apply. Movement of non-wood forest products across international boundaries may be subject to trade restrictions (both tariff and non-tariff). Appropriate authorities should be contacted prior to planned movement of any non-wood forest products across international boundaries. A review of trade restrictions affecting international trade in non-wood forest products may be found in Non-Wood Forest Products No. 8, 1995. The designations employed and the presentation of material in this publication do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. -

China: a Rich Flora Needed of Urgent Conservationprovided by Digital.CSIC
Orsis 19, 2004 49-89 View metadata, citation and similar papers at core.ac.uk brought to you by CORE China: a rich flora needed of urgent conservationprovided by Digital.CSIC López-Pujol, Jordi GReB, Laboratori de Botànica, Facultat de Farmàcia, Universitat de Barcelona, Avda. Joan XXIII s/n, E-08028, Barcelona, Catalonia, Spain. Author for correspondence (E-mail: [email protected]) Zhao, A-Man Laboratory of Systematic and Evolutionary Botany, Institute of Botany, Chinese Academy of Sciences, Beijing 100093, The People’s Republic of China. Manuscript received in april 2004 Abstract China is one of the richest countries in plant biodiversity in the world. Besides to a rich flora, which contains about 33 000 vascular plants (being 30 000 of these angiosperms, 250 gymnosperms, and 2 600 pteridophytes), there is a extraordinary ecosystem diversity. In addition, China also contains a large pool of both wild and cultivated germplasm; one of the eight original centers of crop plants in the world was located there. China is also con- sidered one of the main centers of origin and diversification for seed plants on Earth, and it is specially profuse in phylogenetically primitive taxa and/or paleoendemics due to the glaciation refuge role played by this area in the Quaternary. The collision of Indian sub- continent enriched significantly the Chinese flora and produced the formation of many neoen- demisms. However, the distribution of the flora is uneven, and some local floristic hotspots can be found across China, such as Yunnan, Sichuan and Taiwan. Unfortunately, threats to this biodiversity are huge and have increased substantially in the last 50 years. -

The Chilgoza of Kinnaur. Influence of the Pinus Gerardiana Edible Seed Market Chain Organization on Forest Regeneration in the Indian Himalayas
Original article The Chilgoza of Kinnaur. Influence of the Pinus gerardiana edible seed market chain organization on forest regeneration in the Indian Himalayas Régis PELTIER1, Vincent DAUFFY2 1 Cirad, Es, UR 36, Tac-36 / D, The Chilgoza of Kinnaur. Influence of the Pinus gerardiana edible seed market chain 34398 Montpellier Cedex 5, organization on forest regeneration in the Indian Himalayas. France Abstract –– Context, objective and methods. In the north of India, in the Himalayas, the high-alti- [email protected] tude slopes [(between 1800 and 3300) m] are covered by forests where Pinus gerardiana dominates. This pine is known for its edible seeds (Chilgoza). The recent evolution of nut harvest methods means 2 that there is danger of the disappearance of natural seedlings and the ageing of the forests. Therefore, Inventaire Forestier National a survey was carried out from 1998 with a hundred farmers, which was supplemented with field visits (IFN), Château des Barres, and discussions with resource people involved in the commercial chain. Results. In the 1950s, tradi- 45290 Nogent-sur-Vernisson, tional harvesting rules made it possible to respect trees and to allow a small portion of seeds to reach France the ground. So, in spite of particularly difficult ecological conditions, the forest was able to regenerate. During the five last decades, the roads opening have allowed an irrigated cash-arboriculture develo- [email protected] pment in the valleys. The village communities have become less dependent on the Chilgoza trade and sell the nut harvest contracts to private contractors who employ foreign workers, cut many branches and practically collect all the seeds.