Experimental Study on Facial Nerve Regeneration with Or Without Geniculate Ganglionectomy
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Anatomy-Nerve Tracking
INJECTABLES ANATOMY www.aestheticmed.co.uk Nerve tracking Dr Sotirios Foutsizoglou on the anatomy of the facial nerve he anatomy of the human face has received enormous attention during the last few years, as a plethora of anti- ageing procedures, both surgical and non-surgical, are being performed with increasing frequency. The success of each of those procedures is greatly dependent on Tthe sound knowledge of the underlying facial anatomy and the understanding of the age-related changes occurring in the facial skeleton, ligaments, muscles, facial fat compartments, and skin. The facial nerve is the most important motor nerve of the face as it is the sole motor supply to all the muscles of facial expression and other muscles derived from the mesenchyme in the embryonic second pharyngeal arch.1 The danger zone for facial nerve injury has been well described. Confidence when approaching the nerve and its branches comes from an understanding of its three dimensional course relative to the layered facial soft tissue and being aware of surface anatomy landmarks and measurements as will be discussed in this article. Aesthetic medicine is not static, it is ever evolving and new exciting knowledge emerges every day unmasking the relationship of the ageing process and the macroscopic and microscopic (intrinsic) age-related changes. Sound anatomical knowledge, taking into consideration the natural balance between the different facial structures and facial layers, is fundamental to understanding these changes which will subsequently help us develop more effective, natural, long-standing and most importantly, safer rejuvenating treatments and procedures. The soft tissue of the face is arranged in five layers: 1) Skin; 2) Subcutaneous fat layer; 3) Superficial musculoaponeurotic system (SMAS); 4) Areolar tissue or loose connective tissue (most clearly seen in the scalp and forehead); 5) Deep fascia formed by the periosteum of facial bones and the fascial covering of the muscles of mastication (lateral face). -
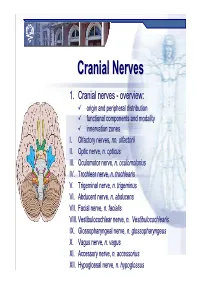
Cranial Nervesnerves
CranialCranial NervesNerves 1. Cranial nerves - overview: origin and peripheral distribution functional components and modality innervation zones I. Olfactory nerves, nn. olfactorii II. Optic nerve, n. opticus III. Oculomotor nerve, n. oculomotorius IV. Trochlear nerve, n. trochlearis V. Trigeminal nerve, n. trigeminus VI. Abducent nerve, n. abducens VII. Facial nerve, n. facialis VIII. Vestibulocochlear nerve, n. Vestibulocochlearis IX. Glossopharyngeal nerve, n. glossopharyngeus X. Vagus nerve, n. vagus XI. Accessory nerve, n. accessorius XII. Hypoglossal nerve, n. hypoglossus Cranial nerves CranialCranial nervesnerves 0. N. terminalis I. N. olfactorius II. N. opticus III. N. oculomotorius IV. N. trochlearis V. N. trigeminus VI. N. abducens VII. N. facialis VIII. N. vestibulocochlearis IX. N. glossopharyngeus X. N. vagus XI. N. accessorius XII. N. hypoglossus Prof. Dr. Nikolai Lazarov 2 Cranial nerves FunctionalFunctional classificationclassification purely sensory (afferent ): n. olfactorius n. opticus n. vestibulocochlearis purely motor (efferent): n. oculomotorius n. trochlearis n. abducens n. accessorius n. hypoglossus mixed (sensory&motor ): n. trigeminus n. facialis n. glossopharyngeus n. vagus autonomic (parasympathetic( ): n. oculomotorius n. facialis n. glossopharyngeus n. vagus Prof. Dr. Nikolai Lazarov 3 Cranial nerves AnatomicAnatomic relationshipsrelationships Location within the brainstem: ventrally: n. olfactorius n. opticus n. oculomotorius n. abducens n. hypoglossus laterally: n. trigeminus -

Surgical Treatment of Bronchial Asthma by Resection of the Laryngeal Nerve
Surgical treatment of bronchial asthma by resection of the laryngeal nerve Ubaidullo Kurbon, Hamza Dodariyon, Abdumalik Davlatov, Sitora Janobilova, Amu Therwath, Massoud Mirshahi To cite this version: Ubaidullo Kurbon, Hamza Dodariyon, Abdumalik Davlatov, Sitora Janobilova, Amu Therwath, et al.. Surgical treatment of bronchial asthma by resection of the laryngeal nerve. BMC Surgery, BioMed Central, 2015, 15 (1), pp.109. 10.1186/s12893-015-0093-2. inserm-01264486 HAL Id: inserm-01264486 https://www.hal.inserm.fr/inserm-01264486 Submitted on 29 Jan 2016 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Kurbon et al. BMC Surgery (2015) 15:109 DOI 10.1186/s12893-015-0093-2 TECHNICAL ADVANCE Open Access Surgical treatment of bronchial asthma by resection of the laryngeal nerve Ubaidullo Kurbon1, Hamza Dodariyon1, Abdumalik Davlatov1, Sitora Janobilova1, Amu Therwath2 and Massoud Mirshahi1,2* Abstract Background: Management of asthma in chronically affected patients is a serious health problem. Our aim was to show that surgical treatment of chronic bronchial asthma by unilateral resection of the internal branch of the superior laryngeal nerve (ib-SLN) is an adequateand lasting remedial response. Patients and methods: In a retrospective study, 41 (26 male and 15 female) patients with bronchial chronic asthma were treated surgically during the period between 2005 and 2013. -

Clinical Anatomy of the Cranial Nerves Clinical Anatomy of the Cranial Nerves
Clinical Anatomy of the Cranial Nerves Clinical Anatomy of the Cranial Nerves Paul Rea AMSTERDAM • BOSTON • HEIDELBERG • LONDON NEW YORK • OXFORD • PARIS • SAN DIEGO SAN FRANCISCO • SINGAPORE • SYDNEY • TOKYO Academic Press is an imprint of Elsevier Academic Press is an imprint of Elsevier 32 Jamestown Road, London NW1 7BY, UK The Boulevard, Langford Lane, Kidlington, Oxford OX5 1GB, UK Radarweg 29, PO Box 211, 1000 AE Amsterdam, The Netherlands 225 Wyman Street, Waltham, MA 02451, USA 525 B Street, Suite 1800, San Diego, CA 92101-4495, USA First published 2014 Copyright r 2014 Elsevier Inc. All rights reserved. No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, or any information storage and retrieval system, without permission in writing from the publisher. Details on how to seek permission, further information about the Publisher’s permissions policies and our arrangement with organizations such as the Copyright Clearance Center and the Copyright Licensing Agency, can be found at our website: www.elsevier.com/permissions. This book and the individual contributions contained in it are protected under copyright by the Publisher (other than as may be noted herein). Notices Knowledge and best practice in this field are constantly changing. As new research and experience broaden our understanding, changes in research methods, professional practices, or medical treatment may become necessary. Practitioners and researchers must always rely on their own experience and knowledge in evaluating and using any information, methods, compounds, or experiments described herein. In using such information or methods they should be mindful of their own safety and the safety of others, including parties for whom they have a professional responsibility. -

Neural Control of Swallowing
AG-2018-48 REVIEW dx.doi.org/10.1590/S0004-2803.201800000-45 Neural control of swallowing Milton Melciades Barbosa COSTA Received 11/4/2018 Accepted 9/5/2018 ABSTRACT – Background – Swallowing is a motor process with several discordances and a very difficult neurophysiological study. Maybe that is the reason for the scarcity of papers about it. Objective – It is to describe the chewing neural control and oral bolus qualification. A review the cranial nerves involved with swallowing and their relationship with the brainstem, cerebellum, base nuclei and cortex was made. Methods – From the reviewed literature including personal researches and new observations, a consistent and necessary revision of concepts was made, not rarely conflicting. Re- sults and Conclusion – Five different possibilities of the swallowing oral phase are described: nutritional voluntary, primary cortical, semiautomatic, subsequent gulps, and spontaneous. In relation to the neural control of the swallowing pharyngeal phase, the stimulus that triggers the pharyngeal phase is not the pharyngeal contact produced by the bolus passage, but the pharyngeal pressure distension, with or without contents. In nutritional swallowing, food and pressure are transferred, but in the primary cortical oral phase, only pressure is transferred, and the pharyngeal response is similar. The pharyngeal phase incorporates, as its functional part, the oral phase dynamics already in course. The pharyngeal phase starts by action of the pharyngeal plexus, composed of the glossopharyngeal (IX), vagus (X) and accessory (XI) nerves, with involvement of the trigeminal (V), facial (VII), glossopharyngeal (IX) and the hypoglossal (XII) nerves. The cervical plexus (C1, C2) and the hypoglossal nerve on each side form the ansa cervicalis, from where a pathway of cervical origin goes to the geniohyoid muscle, which acts in the elevation of the hyoid-laryngeal complex. -

Rhizotomy Targeting the Intermediate Nerve
Zong et al. BMC Surgery 2014, 14:60 http://www.biomedcentral.com/1471-2482/14/60 CASE REPORT Open Access Rhizotomy targeting the intermediate nerve, the glossopharyngeal nerve and the upper 1st to 2nd rootlets of the vagus nerve for the treatment of laryngeal neuralgia combined with intermediate nerve neuralgia-a case report Qiang Zong1, Kai Zhang1*, Guangliang Han1, Shengye Yang2, Lijiang Wang1 and Hongxing Li1 Abstract Background: In neurosurgery, the most common type of facial and pharyngeal pain is trigeminal neuralgia. In contrast, glossopharyngeal neuralgia is relatively rare, and laryngeal neuralgia is the most rarely observed. Case presentation: A case of laryngeal neuralgia combined with intermediate nerve neuralgia that was admitted to our hospital in May 2012 was reported here. The patient was a 58-year-old middle-aged female, who experienced 2 years of paroxysmal burning and stabbing pain near the thyroid perichodrium, in the skin covering the right front side of the neck, and deep in inner ear. Conclusion: The surgical treatment plan similar to that for glossopharyngeal neuralgia could be applied if laryngeal neuralgia is associated with glossopharyngeal neuralgia and intermediate neuralgia or if no obvious improvement is achieved with the above mentioned treatment approaches. Keywords: Laryngeal neuralgia combined with intermediate nerve neuralgia, Trigeminal neuralgia, Rhizotomy Background Case presentation In neurosurgery, the most common type of facial and Written informed consent for participation in the study pharyngeal pain is trigeminal neuralgia. The unbearable was obtained from the participant in this study. The pa- pain caused huge trouble to the patient, and seriously af- tient was a 58-year-old middle-aged female, who experi- fected the quality of patients’ life. -

Elucidate the Pathways Involved in Taste
NAME: ANIH KELECHI FAUSTINA MATRIC NO: 18/MHS02/043 DEPARTMENT: NURSING SCIENCE COLLEGE: COLLEGE OF MEDICINE AND HEALTH SCIENCE. ELUCIDATE THE PATHWAYS INVOLVED IN TASTE: 1. The facial nerve is the seventh cranial nerve, or simply CN VII. It emerges from the pons of the brainstem, controls the muscles of facial expression, and functions in the conveyance of taste sensations from the anterior two-thirds of the tongue. The nerves typically travels from the pons through the facial canal in the temporal bone and exits the skull at the stylomastoid foramen. It arises from the brainstem from an area posterior to the cranial nerve VI (abducens nerve) and anterior to cranial nerve VIII (vestibulocochlear nerve). The facial nerve also supplies preganglionic parasympathetic fibers to several head and neck ganglia. The facial and intermediate nerves can be collectively referred to as the nervus intermediofacialis. - Function Facial expression The main function of the facial nerve is motor control of all of the muscles of facial expression. It also innervates the posterior belly of the digastric muscle, the stylohyoid muscle, and the stapedius muscle of the middle ear. All of these muscles are striated muscles of branchiomeric origin developing from the 2nd pharyngeal arch. Facial sensation In addition, the facial nerve receives taste sensations from the anterior two-thirds of the tongue via the chorda tympani. Taste sensation is sent to the gustatory portion (superior part) of the solitary nucleus. General sensation from the anterior two-thirds of tongue are supplied by afferent fibers of the third division of the fifth cranial nerve (V-3). -

Methodic Materials Cranial Nerves
Medical Academy named after S.I. Georgievsky of V.I. Vernadsky CFU Department of Neurology a n d Neurosurgery Cliiss 4 Anatomy, physiology and pathology of the Cranial nerves VII-XI1 Key points: 1. Brainstem: 1.1. Surface anatomy 1.2.Internal structures and organization 1.3.Cross section through the medulla 1.4.Cross section through the pons 1.5.Cross section through the rostral midbrain 2. Cranial Nerves 2.1. Anatomy 2.2. Functions 2.3. Connections 2.4. Symptoms, signs, syndromes of lesions 2.5. Clinical correlations Key concepts: 1) Study the transverse sections o f the brain stem and localize the cranial nerve nuclei. 2) Study the ventral and dorsal surface o f the brain stem and identify the exiting and entering cranial nerves. 3) Carefully study all o f the figures and legends 4) Study the main information about anatomy of all cranial nerves, their functions, connections and symptoms/signs/syndromes of lesions Define the following terms: Brain stem, medial structures, lateral structures, dorsal structures, tegmentum, medial lemniscus, gracile and cuneate nuclei, pyramids, corticospinal fibers, inferior cerebellar peduncle, spinocerebellar tract, cuneocerebellar tract, olivocerebellar tract, lateral spinothalamic tract (spinal lemniscus), superior colliculi, inferior colliculi, oliva, medial longitudinal fasciculus (MLF), cerebral peduncle, red nucleus, substantia nigra, hypoglossal nucleus o f CNXII, nucleus ambiguus X, andXI), vestibular nuclei (CN VIII,) spinal nucleus and tract o f trigeminal nerve, abducent nucleus of CN VI, facial nucleus (CN VII), spinal nucleus and tract o f trigeminal nerve (CN V), oculomotor nucleus (CN III), cranial nerves I-XII. Literature: Mathias Baehr, M.D., Michael Frotscher, M.D. -

The Functions of the Nervus Intermedius References
The Functions of the Nervus Intermedius into the lesser petrosal nerve entering the otic ganglion. The parotid We read with extreme interest the article written by Burmeister et al gland is, thereafter, reached through the auriculotemporal nerve, a entitled “Identification of the Nervus Intermedius Using 3T MR Im- subdivision of the mandibular subdivision of the trigeminal nerve.6 aging.”1 It is surprising that this minute nerve has gained such a wide In conclusion, we could not find any suggestion that the NI inner- clinical, functional, and now radiologic interest since the original de- vates the parotid gland directly. scription in 1778 by Wrisberg.2 However, there is a point of concern in the article. The authors stated in the introduction that one of the functions of the nervus References intermedius (NI) is the “sensory and parasympathetic innervation of 1. Burmeister HP, Baltzer PA, Dietzel M, et al. Identification of the nervus inter- the parotid gland.” This sentence is somewhat troubling, and we can- medius using 3T MR imaging. AJNR Am J Neuroradiol 2011;32:460–64. Epub not agree with it. 2011 Feb 3 2. Alfieri A, Strauss C, Prell J, et al. History of the nervus intermedius of Wrisberg. To our knowledge, the NI innervates the lacrimal, submandibular, Ann Anat 2010;192:139–44 and sublingual glands. The afferent component of the NI carries sen- 3. Gacek R, Lyon MJ. Evidence of a gustatory-vestibular pathway for protein sorial perception from the skin of the external auditory meatus and transport. Otol Neurotol 2010;31:313–18 4. Scheller C, Rachinger J, Prell J, et al. -
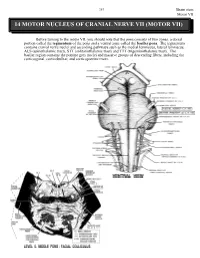
14 Motor Nucleus of Cranial Nerve Vii (Motor Vii)
263 Brain stem Motor VII 14 MOTOR NUCLEUS OF CRANIAL NERVE VII (MOTOR VII) Before turning to the motor VII, you should note that the pons consists of two zones, a dorsal portion called the tegmentum of the pons and a ventral zone called the basilar pons. The tegmentum contains cranial nerve nuclei and ascending pathways such as the medial lemniscus, lateral lemniscus, ALS (spinothalamic tract), STT (solitariothalamic tract) and TTT (trigeminothalamic tract). The basilar region contains the pontine grey nuclei and massive groups of descending fibers, including the corticospinal, corticobulbar, and corticopontine tracts. Brain stem 264 Motor VII The motor nucleus VII contains motor neurons (branchiomotor) that innervate the muscles of facial expression including the orbicularis oculi (CLOSES eyelid), the stapedius, the stylohyoid and the posterior belly of the digastric. Neurons comprising motor VII possess axons that pursue a rather circuitous route in order to exit the brain stem. Initially they pass dorsally and medially to loop over the abducens nucleus. The fibers then course ventrally and laterally to exit the brain stem. The bump in the floor of the fourth ventricle caused by the motor fibers of C.N. VII looping over the abducens nucleus is called the FACIAL COLLICULUS. A unilateral lesion interrupting the axons of C.N. VII results in the following: On the ipsilateral side, the forehead is immobile, the corner of the mouth sags, the nasolabial folds of the face are flattened, facial lines are lost, and saliva may drip from the corner of the mouth. The patient is unable to whistle or puff the cheek because the buccinator muscle is paralyzed. -
Anatomy and Function of the Nervus Intermedius in Stereotactic Radiosurgery for Vestibular Schwannoma
ISSN 1738-6217 REVIEW ARTICLE J of The Kor Soc of Ster and Func Neurosur 2014;10:1-4 Copyright © 2014 The Korean Society of Stereotactic and online © ML Comm Functional Neurosurgery Anatomy and Function of the Nervus Intermedius in Stereotactic Radiosurgery for Vestibular Schwannoma Seong-Hyun Park, MD, PhD Department of Neurosurgery, Kyungpook National University Hospital, Daegu, Korea Complete resection of vestibular schwannomas often presents a major surgical challenge because of the relationship of the tumor to critical neurovascular structures. Stereotactic radiosurgery plays a important role in the management of vestibular schwanno- mas. Some patients undergoing stereotactic radiosurgery (SRS) for vestibular schwannoma experience various disturbances of non-motor components of the facial nerve as a result of the SRS. In this brief review, the author described the anatomy of the ner- vus intermedius, the non-motor component of the facial nerve, and evaluate its dysfunction following stereotactic radiosurgery for the treatment of vestibular schwannoma. KEY WORDS: Gamma Knife radiosurgery · Nervus intermedius · Stereotactic radiosurgery · Vestibular schwannoma. INTRODUCTION vus intermedius during microsurgery or SRS for vestibular schwannoma is necessary to provide better functional out- Stereotactic radiosurgery (SRS) has become an impor- comes and to evaluate various disturbances of non-motor tant alternative option for small to moderate-sized vestibular components of the facial nerve. The facial nerve is a mixed schwannomas since the 1990s. Attention has been usually nerve with both motor and sensory components (nervus in- paid to the excellent rate of tumor control and the preserva- termedius) : motor, parasympathetic, and special sensory tion of the hearing and motor components of the facial nerve (taste). -

Early Development of the Facial Nerve in Human Embryos at Stages 13–15 M
Folia Morphol. Vol. 74, No. 2, pp. 252–257 DOI: 10.5603/FM.2015.0039 O R I G I N A L A R T I C L E Copyright © 2015 Via Medica ISSN 0015–5659 www.fm.viamedica.pl Early development of the facial nerve in human embryos at stages 13–15 M. Weglowski, W. Woźniak, A. Piotrowski, M. Bruska, J. Weglowska, J. Sobański, M. Grzymisławska, J. Łupicka Department of Anatomy, Poznan University of Medical Sciences, Poznan, Poland [Received 6 March 2015; Accepted 17 April 2015] Study was made on 16 human embryos at developmental stages 13–15 (fifth week). The facial nerve was traced on serial sections made in three planes (sagittal, frontal and horizontal) and stained with routine histological methods and impregnated with silver. In embryos at stage 13 the facial ganglion forms a complex structure with the vestibulocochlear ganglion. It is of fusiform shape in contact with epipharyngeal placode and is located anteriorly and ventrally to the vestibulocochlear ganglion. In embryos at stage 14 the facial ganglion separates from the vestibular and cochlear ganglia and the chorda tympani as the first branch appears. During stage 15 the main trunk of the facial nerve elongates and the greater petrosal nerve originates at the level of the facial ganglion and above the origin of the chorda tympani. (Folia Morphol 2015; 74, 2: 252–257) Key words: human embryonic period, nervous system, facial nerve INTRODUCTION 21, 33]. The sensory fibres of the intermediate nerve The facial nerve is a second pharyngeal arch nerve comprise the greater part of this nerve.