Elucidate the Pathways Involved in Taste
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Neuroanatomy Crash Course
Neuroanatomy Crash Course Jens Vikse ∙ Bendik Myhre ∙ Danielle Mellis Nilsson ∙ Karoline Hanevik Illustrated by: Peder Olai Skjeflo Holman Second edition October 2015 The autonomic nervous system ● Division of the autonomic nervous system …………....……………………………..………….…………... 2 ● Effects of parasympathetic and sympathetic stimulation…………………………...……...……………….. 2 ● Parasympathetic ganglia ……………………………………………………………...…………....………….. 4 Cranial nerves ● Cranial nerve reflexes ………………………………………………………………….…………..…………... 7 ● Olfactory nerve (CN I) ………………………………………………………………….…………..…………... 7 ● Optic nerve (CN II) ……………………………………………………………………..…………...………….. 7 ● Pupillary light reflex …………………………………………………………………….…………...………….. 7 ● Visual field defects ……………………………………………...................................…………..………….. 8 ● Eye dynamics …………………………………………………………………………...…………...………….. 8 ● Oculomotor nerve (CN III) ……………………………………………………………...…………..………….. 9 ● Trochlear nerve (CN IV) ………………………………………………………………..…………..………….. 9 ● Trigeminal nerve (CN V) ……………………………………………………................…………..………….. 9 ● Abducens nerve (CN VI) ………………………………………………………………..…………..………….. 9 ● Facial nerve (CN VII) …………………………………………………………………...…………..………….. 10 ● Vestibulocochlear nerve (CN VIII) …………………………………………………….…………...…………. 10 ● Glossopharyngeal nerve (CN IX) …………………………………………….……….…………...………….. 10 ● Vagus nerve (CN X) …………………………………………………………..………..…………...………….. 10 ● Accessory nerve (CN XI) ……………………………………………………...………..…………..………….. 11 ● Hypoglossal nerve (CN XII) …………………………………………………..………..…………...…………. -

Anatomy-Nerve Tracking
INJECTABLES ANATOMY www.aestheticmed.co.uk Nerve tracking Dr Sotirios Foutsizoglou on the anatomy of the facial nerve he anatomy of the human face has received enormous attention during the last few years, as a plethora of anti- ageing procedures, both surgical and non-surgical, are being performed with increasing frequency. The success of each of those procedures is greatly dependent on Tthe sound knowledge of the underlying facial anatomy and the understanding of the age-related changes occurring in the facial skeleton, ligaments, muscles, facial fat compartments, and skin. The facial nerve is the most important motor nerve of the face as it is the sole motor supply to all the muscles of facial expression and other muscles derived from the mesenchyme in the embryonic second pharyngeal arch.1 The danger zone for facial nerve injury has been well described. Confidence when approaching the nerve and its branches comes from an understanding of its three dimensional course relative to the layered facial soft tissue and being aware of surface anatomy landmarks and measurements as will be discussed in this article. Aesthetic medicine is not static, it is ever evolving and new exciting knowledge emerges every day unmasking the relationship of the ageing process and the macroscopic and microscopic (intrinsic) age-related changes. Sound anatomical knowledge, taking into consideration the natural balance between the different facial structures and facial layers, is fundamental to understanding these changes which will subsequently help us develop more effective, natural, long-standing and most importantly, safer rejuvenating treatments and procedures. The soft tissue of the face is arranged in five layers: 1) Skin; 2) Subcutaneous fat layer; 3) Superficial musculoaponeurotic system (SMAS); 4) Areolar tissue or loose connective tissue (most clearly seen in the scalp and forehead); 5) Deep fascia formed by the periosteum of facial bones and the fascial covering of the muscles of mastication (lateral face). -
Cranial Nervesnerves
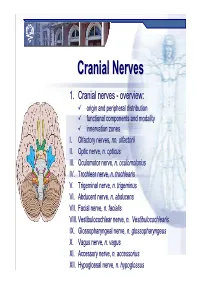
CranialCranial NervesNerves 1. Cranial nerves - overview: origin and peripheral distribution functional components and modality innervation zones I. Olfactory nerves, nn. olfactorii II. Optic nerve, n. opticus III. Oculomotor nerve, n. oculomotorius IV. Trochlear nerve, n. trochlearis V. Trigeminal nerve, n. trigeminus VI. Abducent nerve, n. abducens VII. Facial nerve, n. facialis VIII. Vestibulocochlear nerve, n. Vestibulocochlearis IX. Glossopharyngeal nerve, n. glossopharyngeus X. Vagus nerve, n. vagus XI. Accessory nerve, n. accessorius XII. Hypoglossal nerve, n. hypoglossus Cranial nerves CranialCranial nervesnerves 0. N. terminalis I. N. olfactorius II. N. opticus III. N. oculomotorius IV. N. trochlearis V. N. trigeminus VI. N. abducens VII. N. facialis VIII. N. vestibulocochlearis IX. N. glossopharyngeus X. N. vagus XI. N. accessorius XII. N. hypoglossus Prof. Dr. Nikolai Lazarov 2 Cranial nerves FunctionalFunctional classificationclassification purely sensory (afferent ): n. olfactorius n. opticus n. vestibulocochlearis purely motor (efferent): n. oculomotorius n. trochlearis n. abducens n. accessorius n. hypoglossus mixed (sensory&motor ): n. trigeminus n. facialis n. glossopharyngeus n. vagus autonomic (parasympathetic( ): n. oculomotorius n. facialis n. glossopharyngeus n. vagus Prof. Dr. Nikolai Lazarov 3 Cranial nerves AnatomicAnatomic relationshipsrelationships Location within the brainstem: ventrally: n. olfactorius n. opticus n. oculomotorius n. abducens n. hypoglossus laterally: n. trigeminus -

ANATOMY of EAR Basic Ear Anatomy
ANATOMY OF EAR Basic Ear Anatomy • Expected outcomes • To understand the hearing mechanism • To be able to identify the structures of the ear Development of Ear 1. Pinna develops from 1st & 2nd Branchial arch (Hillocks of His). Starts at 6 Weeks & is complete by 20 weeks. 2. E.A.M. develops from dorsal end of 1st branchial arch starting at 6-8 weeks and is complete by 28 weeks. 3. Middle Ear development —Malleus & Incus develop between 6-8 weeks from 1st & 2nd branchial arch. Branchial arches & Development of Ear Dev. contd---- • T.M at 28 weeks from all 3 germinal layers . • Foot plate of stapes develops from otic capsule b/w 6- 8 weeks. • Inner ear develops from otic capsule starting at 5 weeks & is complete by 25 weeks. • Development of external/middle/inner ear is independent of each other. Development of ear External Ear • It consists of - Pinna and External auditory meatus. Pinna • It is made up of fibro elastic cartilage covered by skin and connected to the surrounding parts by ligaments and muscles. • Various landmarks on the pinna are helix, antihelix, lobule, tragus, concha, scaphoid fossa and triangular fossa • Pinna has two surfaces i.e. medial or cranial surface and a lateral surface . • Cymba concha lies between crus helix and crus antihelix. It is an important landmark for mastoid antrum. Anatomy of external ear • Landmarks of pinna Anatomy of external ear • Bat-Ear is the most common congenital anomaly of pinna in which antihelix has not developed and excessive conchal cartilage is present. • Corrections of Pinna defects are done at 6 years of age. -

Surgical Treatment of Bronchial Asthma by Resection of the Laryngeal Nerve
Surgical treatment of bronchial asthma by resection of the laryngeal nerve Ubaidullo Kurbon, Hamza Dodariyon, Abdumalik Davlatov, Sitora Janobilova, Amu Therwath, Massoud Mirshahi To cite this version: Ubaidullo Kurbon, Hamza Dodariyon, Abdumalik Davlatov, Sitora Janobilova, Amu Therwath, et al.. Surgical treatment of bronchial asthma by resection of the laryngeal nerve. BMC Surgery, BioMed Central, 2015, 15 (1), pp.109. 10.1186/s12893-015-0093-2. inserm-01264486 HAL Id: inserm-01264486 https://www.hal.inserm.fr/inserm-01264486 Submitted on 29 Jan 2016 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Kurbon et al. BMC Surgery (2015) 15:109 DOI 10.1186/s12893-015-0093-2 TECHNICAL ADVANCE Open Access Surgical treatment of bronchial asthma by resection of the laryngeal nerve Ubaidullo Kurbon1, Hamza Dodariyon1, Abdumalik Davlatov1, Sitora Janobilova1, Amu Therwath2 and Massoud Mirshahi1,2* Abstract Background: Management of asthma in chronically affected patients is a serious health problem. Our aim was to show that surgical treatment of chronic bronchial asthma by unilateral resection of the internal branch of the superior laryngeal nerve (ib-SLN) is an adequateand lasting remedial response. Patients and methods: In a retrospective study, 41 (26 male and 15 female) patients with bronchial chronic asthma were treated surgically during the period between 2005 and 2013. -

Atlas of the Facial Nerve and Related Structures
Rhoton Yoshioka Atlas of the Facial Nerve Unique Atlas Opens Window and Related Structures Into Facial Nerve Anatomy… Atlas of the Facial Nerve and Related Structures and Related Nerve Facial of the Atlas “His meticulous methods of anatomical dissection and microsurgical techniques helped transform the primitive specialty of neurosurgery into the magnificent surgical discipline that it is today.”— Nobutaka Yoshioka American Association of Neurological Surgeons. Albert L. Rhoton, Jr. Nobutaka Yoshioka, MD, PhD and Albert L. Rhoton, Jr., MD have created an anatomical atlas of astounding precision. An unparalleled teaching tool, this atlas opens a unique window into the anatomical intricacies of complex facial nerves and related structures. An internationally renowned author, educator, brain anatomist, and neurosurgeon, Dr. Rhoton is regarded by colleagues as one of the fathers of modern microscopic neurosurgery. Dr. Yoshioka, an esteemed craniofacial reconstructive surgeon in Japan, mastered this precise dissection technique while undertaking a fellowship at Dr. Rhoton’s microanatomy lab, writing in the preface that within such precision images lies potential for surgical innovation. Special Features • Exquisite color photographs, prepared from carefully dissected latex injected cadavers, reveal anatomy layer by layer with remarkable detail and clarity • An added highlight, 3-D versions of these extraordinary images, are available online in the Thieme MediaCenter • Major sections include intracranial region and skull, upper facial and midfacial region, and lower facial and posterolateral neck region Organized by region, each layered dissection elucidates specific nerves and structures with pinpoint accuracy, providing the clinician with in-depth anatomical insights. Precise clinical explanations accompany each photograph. In tandem, the images and text provide an excellent foundation for understanding the nerves and structures impacted by neurosurgical-related pathologies as well as other conditions and injuries. -

Dissection and Exposure of the Whole Course of Deep Nerves in Human Head Specimens After Decalcification
Hindawi Publishing Corporation International Journal of Otolaryngology Volume 2012, Article ID 418650, 7 pages doi:10.1155/2012/418650 Research Article Dissection and Exposure of the Whole Course of Deep Nerves in Human Head Specimens after Decalcification Longping Liu, Robin Arnold, and Marcus Robinson Discipline of Anatomy and Histology, University of Sydney, Anderson Stuart Building F13, Sydney, NSW 2006, Australia Correspondence should be addressed to Marcus Robinson, [email protected] Received 29 July 2011; Revised 10 November 2011; Accepted 12 December 2011 AcademicEditor:R.L.Doty Copyright © 2012 Longping Liu et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. The whole course of the chorda tympani nerve, nerve of pterygoid canal, and facial nerves and their relationships with surrounding structures are complex. After reviewing the literature, it was found that details of the whole course of these deep nerves are rarely reported and specimens displaying these nerves are rarely seen in the dissecting room, anatomical museum, or atlases. Dissections were performed on 16 decalcified human head specimens, exposing the chorda tympani and the nerve connection between the geniculate and pterygopalatine ganglia. Measurements of nerve lengths, branching distances, and ganglia size were taken. The chorda tympani is a very fine nerve (0.44 mm in diameter within the tympanic cavity) and approximately 54 mm in length. The mean length of the facial nerve from opening of internal acoustic meatus to stylomastoid foramen was 52.5 mm. -

Clinical Anatomy of the Cranial Nerves Clinical Anatomy of the Cranial Nerves
Clinical Anatomy of the Cranial Nerves Clinical Anatomy of the Cranial Nerves Paul Rea AMSTERDAM • BOSTON • HEIDELBERG • LONDON NEW YORK • OXFORD • PARIS • SAN DIEGO SAN FRANCISCO • SINGAPORE • SYDNEY • TOKYO Academic Press is an imprint of Elsevier Academic Press is an imprint of Elsevier 32 Jamestown Road, London NW1 7BY, UK The Boulevard, Langford Lane, Kidlington, Oxford OX5 1GB, UK Radarweg 29, PO Box 211, 1000 AE Amsterdam, The Netherlands 225 Wyman Street, Waltham, MA 02451, USA 525 B Street, Suite 1800, San Diego, CA 92101-4495, USA First published 2014 Copyright r 2014 Elsevier Inc. All rights reserved. No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, or any information storage and retrieval system, without permission in writing from the publisher. Details on how to seek permission, further information about the Publisher’s permissions policies and our arrangement with organizations such as the Copyright Clearance Center and the Copyright Licensing Agency, can be found at our website: www.elsevier.com/permissions. This book and the individual contributions contained in it are protected under copyright by the Publisher (other than as may be noted herein). Notices Knowledge and best practice in this field are constantly changing. As new research and experience broaden our understanding, changes in research methods, professional practices, or medical treatment may become necessary. Practitioners and researchers must always rely on their own experience and knowledge in evaluating and using any information, methods, compounds, or experiments described herein. In using such information or methods they should be mindful of their own safety and the safety of others, including parties for whom they have a professional responsibility. -

In Vivo Neurophysiological Recordings From
University of Nebraska at Omaha DigitalCommons@UNO Psychology Faculty Publications Department of Psychology 2005 In vivo neurophysiological recordings from geniculate ganglia: taste response properties of individual greater superficial petrosal and chorda tympani neurones Suzanne I. Sollars University of Nebraska at Omaha, [email protected] David L. Hill University of Virginia Follow this and additional works at: https://digitalcommons.unomaha.edu/psychfacpub Part of the Psychology Commons Recommended Citation Sollars, Suzanne I. and Hill, David L., "In vivo neurophysiological recordings from geniculate ganglia: taste response properties of individual greater superficial petrosal and chorda tympani neurones" (2005). Psychology Faculty Publications. 227. https://digitalcommons.unomaha.edu/psychfacpub/227 This Article is brought to you for free and open access by the Department of Psychology at DigitalCommons@UNO. It has been accepted for inclusion in Psychology Faculty Publications by an authorized administrator of DigitalCommons@UNO. For more information, please contact [email protected]. tjp˙828 TJP-xml.cls March14, 2005 20:32 JPhysiol 000.0 (2005) pp 1–17 1 In vivo neurophysiological recordings from geniculate ganglia: taste response properties of individual greater superficial petrosal and chorda tympani neurones Suzanne I. Sollars1 and David L. Hill2 1Department of Psychology, University of Nebraska Omaha, Omaha, NE 68182, USA 2Department of Psychology, University of Virginia, Charlottesville, VA 22904, USA Coding of gustatory information is complex and unique among sensory systems; information is received by multiple receptor populations located throughout the oral cavity and carried to a single central relay by four separate nerves. The geniculate ganglion is the location of the somata of two of these nerves, the greater superficial petrosal (GSP) and the chorda tympani (CT). -

Neural Control of Swallowing
AG-2018-48 REVIEW dx.doi.org/10.1590/S0004-2803.201800000-45 Neural control of swallowing Milton Melciades Barbosa COSTA Received 11/4/2018 Accepted 9/5/2018 ABSTRACT – Background – Swallowing is a motor process with several discordances and a very difficult neurophysiological study. Maybe that is the reason for the scarcity of papers about it. Objective – It is to describe the chewing neural control and oral bolus qualification. A review the cranial nerves involved with swallowing and their relationship with the brainstem, cerebellum, base nuclei and cortex was made. Methods – From the reviewed literature including personal researches and new observations, a consistent and necessary revision of concepts was made, not rarely conflicting. Re- sults and Conclusion – Five different possibilities of the swallowing oral phase are described: nutritional voluntary, primary cortical, semiautomatic, subsequent gulps, and spontaneous. In relation to the neural control of the swallowing pharyngeal phase, the stimulus that triggers the pharyngeal phase is not the pharyngeal contact produced by the bolus passage, but the pharyngeal pressure distension, with or without contents. In nutritional swallowing, food and pressure are transferred, but in the primary cortical oral phase, only pressure is transferred, and the pharyngeal response is similar. The pharyngeal phase incorporates, as its functional part, the oral phase dynamics already in course. The pharyngeal phase starts by action of the pharyngeal plexus, composed of the glossopharyngeal (IX), vagus (X) and accessory (XI) nerves, with involvement of the trigeminal (V), facial (VII), glossopharyngeal (IX) and the hypoglossal (XII) nerves. The cervical plexus (C1, C2) and the hypoglossal nerve on each side form the ansa cervicalis, from where a pathway of cervical origin goes to the geniohyoid muscle, which acts in the elevation of the hyoid-laryngeal complex. -

Damage to Taste (Otitis Media) Is Associated with Dysgeusia, Intensified Pain Experience and Weight Gain
DAMAGE TO TASTE (OTITIS MEDIA) IS ASSOCIATED WITH DYSGEUSIA, INTENSIFIED PAIN EXPERIENCE AND WEIGHT GAIN L.M. Bartoshuk1, F.A. Catalanotto1, V.B. Duffy2, H. Hoffman3, H. Logan1, V. Mayo1 and D.J. Snyder1,4 1University of Florida Smell & Taste Center, 2 Allied Health, University of Connecticut, 3NIDCD, 4Neuroscience, Yale University ABSTRACT METHODS DISCUSSION The chorda tympani taste nerve traverses the middle ear and may be damaged by Data analyzed are from a questionnaire study initiated in 1993. Lecture attendees (cumulative Dysgeusia and Strongest Oral Pain. Using anesthesia of taste nerves along with study of infections (otitis media). Lecture attendees (N=6984) answered questions (Have you total about 6500) were asked to provide demographic information (age, sex, height, weight) as well as patients with taste damage, we have hypothesized that loss of taste input releases inhibition ever suffered from middle ear infections? Do you ever have persistent salty, sweet, information about histories of otitis media and dysgeusia: producing phantom sensations as well as intensifying sensations evoked by some oral stimuli. sour, or bitter tastes in your mouth?) and reported height and weight. Analyses were The observation that dysgeusia (chronic taste sensations in the absence of stimulation) and Have you ever suffered from middle ear infections? done with multiple regression (controlling for sex and age). Those with histories of intensified oral pain are associated with a history of otitis media is consistent with the damage to (1) no, (2) yes, but not serious, (3) yes, required antibiotics more than once, otitis media reported more dysgeusia and had higher BMIs (weight corrected for taste inflicted by otitis media. -

Superior Laryngeal Nerve Response Patterns to Chemical Stimulation of Sheep Epiglottis
Brain Research, 276 (1983) 81-93 81 Elsevier Superior Laryngeal Nerve Response Patterns to Chemical Stimulation of Sheep Epiglottis ROBERT M. BRADLEY 1,2,* HAZEL M. STEDMAN 1 and CHARLOTTE M. MISTREqTA 1.3 1Department of Oral Biology, School of Dentistry University of Michigan, Ann Arbor, M1 48109, 2Department of Physiology, School of Medicine, Universityof Michigan, Ann Arbor, M148109, and 3Centerfor Human Growth and Development, and The Research Center, School of Nursing, Universityof Michigan, Ann Arbor, M148109 (U.S.A.) (Accepted February 8th, 1983) Key words: epiglottis -- superior laryngeal nerve -- chemoreception -- neurophysiology-- sheep Responses were recorded from single fibers of the sheep superior laryngeal nerve during stimulation of the epiglottis with 0.5 M KCI, NH4CI, NaC1 and LiCI, distilled water, 0.005 M citric acid, and 0.01 N HCI. Recordings were made from both lambs and ewes. KCI elicited a response from 99% of fibers followed in order of effective stimulation by NH4CI, HCI, distilled water, citric acid, NaCI and LiC1. Analysis of the variation in response frequency with time demonstrated differences in the response patterns for these stimu- li. The pattern of frequency over time is sufficient to discriminate among the salts, between some of the salts and acids, and between some of the salts and water. Therefore the response pattern may be significant in initiating the various reflex activities that occur dur- ing chemical stimulation of the larynx. INTRODUCTION rious that taste scientists have virtually ignored the study of chemosensitive responses from the SLN. Sensory responses from the superior laryngeal Recently, however, Stedman et al. 23 investigated nerve (SLN) have been studied to investigate, pri- chemosensitive fibers supplying epiglottal taste buds marily, the neural mechanisms underlying upper air- in cats, using a variety of salt and acid stimuli, and way reflexes.