In Vivo Neurophysiological Recordings From
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Absence of Bone Over the Geniculate Ganglion
Absence of Bone over the Geniculate Ganglion ALBERT L. RHOTON, JR., M.D., JACK L. PULEC, M.D., GEORGE M. HALL, M.D., AND ALLEN S. BOYD, JR., M.D. Sections of Neurosurgical Research and Otolaryngology, Mayo Clinic and Mayo Foundation, and Mayo Graduate School of Medicine, Rochester, Minnesota ANDY recognized that the geniculate TABLE 1 ganglion "occasionally" protrudes Frequency and amount of exposure of geniculate D through a congenital defect in the roof ganglion and genu of the seventh nerve of the petrous temporal bone. In their book on in 50 autopsy examinations trigeminal neuralgia, Stookey and Ransohoff16 noted that in rare instances the petrous bone is Amount exposed (mm) Number defective over the geniculate ganglion. This fact has been considered of such minor sig- 0.54).9 2 1.0-1.9 5 nificance that it is largely disregarded in de- 2.0-2.9 6 scriptions of neurosurgical procedures for 3.0-4.0 2 treatment of trigeminal neuralgia in which the roof of the petrous temporal bone may be ex- Total 15 posed, and it is not among the anatomical variants listed in standard anatomy text- graphs were taken with the original paint books, 9,x3 although it has been mentioned in intact to illustrate the extent of exposure of the otological literature. House and Crabtree 1~ the nerve (Figs. 1-4). reported the incidence of this bony anomaly to be 5 %, presumably on the basis of their clin- Results ical material. Batson3 stated that the genicu- In 15 bones (15 %), all or part of the genicu- late ganglion is not covered by bone in early late ganglion and genu of the seventh nerve childhood. -

Cranial and Spinal Nerve Anastomoses in Man
MOJ Anatomy & Physiology Mini Review Open Access Cranial and spinal nerve anastomoses in man Abstract Volume 7 Issue 4 - 2020 Nerve anastomoses normally exist in human body between cranial nerves, spinal nerves, Heshmat SW Haroun and between cranial and spinal nerves. They are also frequently performed in surgeries for Department of Anatomy, Cairo University, Egypt recovery of functions of denervated areas. These anastomoses are of utmost importance in the process of reinnervation and nerve repair following individual nerve injury. Examples Correspondence: Heshmat SW Haroun, Professor of Anatomy of cranial nerve anastomoses are demonstrated between the branches of the facial nerve and and Embryology, Anatomy Department, Kasr Al Ainy Faculty of other nerves, the nerves of the tongue, the laryngeal nerves of the vagus nerve, and the optic Medicine, Cairo University, Egypt, nerves. Examples of spinal nerve anastomoses are also displayed between the spinal nerve Email roots and between the individual nerves of the different somatic nerve plexuses: cervical, brachial, lumbar, and sacral. Received: June 18, 2020 | Published: July 07, 2020 Keywords: nerve anastomoses, cranial nerves, spinal nerves Introduction the cervical branch of the intact facial nerve was anastomosed to the temporal branch of the paralyzed facial nerve using a 20cm- long Nerve anastomoses indicate interconnections between the branches sural nerve autograft or the ipsilateral descendens cervicalis (of ansa of different nerves. They are also surgically done to reinnervate nerve- cervicalis) was anastomosed to the cervical branch of the paralyzed deprived regions. The incidence of these anastomoses may alter the facial nerve.6 clinical and electrophysiological manifestations of different muscular disorders, modify the required surgical procedures, or may be a source Anastomoses between the facial nerve and other nerves were of iatrogenic nerve injury. -

Anatomy of the Ear
Anatomy of the Ear Neuroanatomy block-Anatomy-Lecture 10 Editing file Objectives At the end of the lecture, students should be able to: ● List the parts of the ear: External, Middle (tympanic cavity) and Internal (labyrinth). ● Describe the parts of the external ear: auricle and external auditory meatus. ● Identify the boundaries of the middle ear : roof, floor and four walls (anterior, posterior, medial and lateral). ● Define the contents of the tympanic cavity: I. Ear ossicles (malleus, incus, and stapes) II. Muscles (tensor tympani and stapedius III. Nerves (branches of facial and glossopharyngeal) ● List the parts of the inner ear,bony part filled with perilymph (cochlea, vestibule, and semicircular canals), in which is suspended the membranous part that is filled with Color guide endolymph ● Only in boys slides in Green ● List the organs of hearing and equilibrium ● Only in girls slides in Purple ● important in Red ● Notes in Grey The External Ear Formed By The External Auditory The Auricle Canal ● It has a characteristic shape ● is a curved S-shaped tube about and it collects air vibrations 2.5 cm, that conducts & collects ● It consists of a thin plate of sound waves from the auricle to elastic cartilage covered by a the tympanic membrane. Its double layer of skin outer 1/3rd is elastic cartilage, ● It receives the insertion of Tympanic while its inner 2/3rds are bony extrinsic muscles which are membrane ● Its lined by skin, and its outer supplied by the facial nerve. External 1/3rd is provided with hairs, Sensation is carried by acoustic meatus sebaceous and ceruminous greater auricular & glands (modified sweat glands auriculotemporal nerves that secrete a yellowish brownish substance called ear wax) * The auricle is also called pinna * The external auditory canal is also called the external auditory (acoustic) meatus 3 Middle Ear (Tympanic Cavity) ● The middle ear is a narrow, oblique slit-like cavity (air-filled) in the petrous temporal bone & lined with mucous membrane. -

Elucidate the Pathways Involved in Taste
NAME: ANIH KELECHI FAUSTINA MATRIC NO: 18/MHS02/043 DEPARTMENT: NURSING SCIENCE COLLEGE: COLLEGE OF MEDICINE AND HEALTH SCIENCE. ELUCIDATE THE PATHWAYS INVOLVED IN TASTE: 1. The facial nerve is the seventh cranial nerve, or simply CN VII. It emerges from the pons of the brainstem, controls the muscles of facial expression, and functions in the conveyance of taste sensations from the anterior two-thirds of the tongue. The nerves typically travels from the pons through the facial canal in the temporal bone and exits the skull at the stylomastoid foramen. It arises from the brainstem from an area posterior to the cranial nerve VI (abducens nerve) and anterior to cranial nerve VIII (vestibulocochlear nerve). The facial nerve also supplies preganglionic parasympathetic fibers to several head and neck ganglia. The facial and intermediate nerves can be collectively referred to as the nervus intermediofacialis. - Function Facial expression The main function of the facial nerve is motor control of all of the muscles of facial expression. It also innervates the posterior belly of the digastric muscle, the stylohyoid muscle, and the stapedius muscle of the middle ear. All of these muscles are striated muscles of branchiomeric origin developing from the 2nd pharyngeal arch. Facial sensation In addition, the facial nerve receives taste sensations from the anterior two-thirds of the tongue via the chorda tympani. Taste sensation is sent to the gustatory portion (superior part) of the solitary nucleus. General sensation from the anterior two-thirds of tongue are supplied by afferent fibers of the third division of the fifth cranial nerve (V-3). -

Neural Connections Between the Nervus Intermedius and the Facial and Vestibulocochlear
Re-Submission for Surg Radiol Anat, October 5, 2015 Neural Connections between the Nervus Intermedius and the Facial and Vestibulocochlear Nerves in the Cerebellopontine Angle: An Anatomic Study R. Shane Tubbs1, MS, PA-C, PhD, Nicole Hose1, Marios Loukas2, MD, PhD, Raffaele De Caro3, PhD, Aaron A. Cohen-Gadol4,5, MD, MSc 1Seattle Science Foundation, Seattle, WA, USA 2Department of Anatomical Sciences, St. George’s University, Grenada 3 Institute of Anatomy, University of Padova, Padova, Italy 4Goodman Campbell Brain and Spine, Department of Neurological Surgery, Indiana University School of Medicine, Indianapolis, Indiana 5. Indiana University Simon Cancer Center, Indianapolis, Indiana E-mail addresses: R. Shane Tubbs: [email protected] Nicole Hose: [email protected] Marios Loukas: [email protected] Raffaele De Caro: [email protected] Aaron A. Cohen-Gadol: [email protected] Correspondence: Aaron A. Cohen-Gadol, MD, MSc __________________________________________________________________________________________ This is the author's manuscript of the article published in final edited form as: Shane Tubbs, R., Hose, N., Loukas, M., de Caro, R., & Cohen-Gadol, A. A. (2015). Neural connections between the nervus intermedius and the facial and vestibulocochlear nerves in the cerebellopontine angle. Surgical and Radiologic Anatomy. http://dx.doi.org/10.1007/s00276-015-1571-z Goodman Campbell Brain and Spine Department of Neurological Surgery Indiana University School of Medicine 355 W. 16th Street, Suite 5100 Indianapolis, IN 46202 Phone: 317-362-8760 Fax: 317-396-1280 E-mail: [email protected] Funding and conflicts of interest: None Key words: anatomy; surgery; skull base; iatrogenic injury; ramus communicans; internal auditory meatus; facial nerve; nervus intermedius; vestibulocochlear nerve 2 Abstract Purpose: Unexpected clinical outcomes following transection of single nerves of the internal acoustic meatus have been reported. -

Facial Nerve Preservation in Geniculate Ganglion Hemangiomas
Acta Oto-Laryngologica. 2014; 134: 974–976 ORIGINAL ARTICLE Facial nerve preservation in geniculate ganglion hemangiomas XIAOFENG MA1, DONG CHEN1, LI CAI1 & DAOWEN WANG2 1Department of Otolaryngology Head and Neck Surgery, The First Affiliated Hospital of Liaoning Medical University, Jinzhou and 2Department of Otolaryngology Head and Neck Surgery, Peking University Health Science Center, Beijing, PR China Abstract Conclusions: Facial nerve preservation was related to tumor size, and the patients with facial nerve preservation obtained better recovery. Hence it is necessary to perform surgical removal as soon as possible. Objective: To study facial nerve preservation in patients with geniculate ganglion (GG) hemangiomas. Methods: Twelve patients who had GG hemangiomas were managed at a single institute. All patients underwent total tumor removal, and the surgeon attempted to preserve the facial nerve. Tumor size was measured by MRI, and the patients were divided into two groups according to tumor size: larger size group (‡10 mm) and smaller size group (<10 mm). Results: Generally, the facial nerve was successfully preserved in 10 of 12 cases (83.30%), and nerve grafting was required in 2 cases. Seven of 10 patients (70%) with nerve intact recovered to grade I or grade II, while the 2 cases with nerve grafting recovered to grade III or grade IV. Among the smaller size group, the facial nerves of all patients (100%) were intact. In contrast, only one of three patients (33.3%) in the larger size group maintained nerve integrity after surgery. Keywords: Facial palsy, nerve graft, nerve deficit For personal use only. Introduction Material and methods Geniculate ganglion (GG) hemangiomas were first A consecutive case series of 12 patients who had GG described by Pulec in 1969 [1]. -

Anatomical Features of Intratemporal Course of Facial Nerve and Its Variations
Open Access Original Article DOI: 10.7759/cureus.3085 Anatomical Features of Intratemporal Course of Facial Nerve and its Variations Raja Kalaiarasi 1 , Avvaru Satya Kiran 2 , Chellappa Vijayakumar 3 , Ramakrishnan Venkataramanan 4 , Manusrut Manusrut 5 , Ravi Prabhu 6 1. Otorhinolaryngology, Sri Lakshmi Narayana Institute of Medical Science, Puducherry, IND 2. Otolaryngology, Rainbow Children's Hospital, Hyderabad, IND 3. Surgery, Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry, IND 4. Otolaryngology, Sri Lakshmi Narayana Institute of Medical Science, Pondicherry, IND 5. Otolaryngology, ESIC Medical College, Hyderabad , IND 6. General Surgery, Sri Lakshmi Narayana Institute of Medical Science, Pondicherry, IND Corresponding author: Raja Kalaiarasi, [email protected] Abstract Introduction Facial nerve has the longest and complex course in its bony canal. The anatomical variations make the nerve prone to injury during mastoid surgeries. Having a thorough anatomical knowledge and its variations is must for the surgeons to avoid injury to this vital structure and for the safe surgery. The objective of the study was to describe the anatomical variations of intratemporal portion of facial nerve. Materials and methods The study was conducted in the department of otorhinolaryngology in the temporal bone dissection laboratory of a tertiary health care centre. Fifty wet temporal bones were dissected by the same team of surgeons for the duration of one year to study the various anomalies of the intratemporal course of the facial nerve and its relations with the other important middle ear structures. Results The mean length of the labyrinth, tympanic and mastoid segment of the facial nerve was 4.1 mm (±0.6 mm), 9.34 mm (±1.12 mm) and 12.8 mm (±1.8 mm), respectively. -

Atypical Tumors of the Facial Nerve: Case Series and Review of the Literature
Neurosurg Focus 34 (3):E2, 2013 ©AANS, 2013 Atypical tumors of the facial nerve: case series and review of the literature LINDSEY ROSS, M.D.,1 DONIEL DRAZIN, M.D.,1 PAULA EBOLI, M.D.,1 AND GREGORY P. LEKOVIC, M.D., PH.D.1,2 1Department of Neurosurgery, Cedars-Sinai Medical Center; and 2House Clinic/House Research Institute, Los Angeles, California Object. The authors present a series of 4 patients with rare facial nerve tumors. The relevant literature is re viewed and is discussed regarding diagnostic features, the role of operative management, and surgical approach. Methods. A retrospective chart review was conducted for patients with tumors of the facial nerve that were treated between 2008 and 2011. Patients undergoing observation with serial MRI and those who were treated with upfront radiosurgery and for whom tissue diagnosis was not available were excluded. In addition, patients with suspected vestibular schwannoma, facial nerve schwannoma, neurofibromatosis Type 2, and metastatic disease were also excluded. The charts of 4 patients (2 men and 2 women) with “atypical” tumors were reviewed and analyzed. Results. A total of 12 patients with tumors of the facial nerve were identified during the study period. Patient characteristics, preoperative imaging, operative approach, tumor histology, and outcomes are described. Conclusions. Atypical facial nerve tumors must be distinguished from the more common facial nerve schwan noma. How the authors of this study treat rare facial nerve tumors is based on their experience with the more common facial nerve schwannomas, characterized by a slow progression of symptoms and growth. Less is known about the rare lesions, and thus a conservative approach may be warranted. -

A Review of Facial Nerve Anatomy
A Review of Facial Nerve Anatomy Terence M. Myckatyn, M.D.1 and Susan E. Mackinnon, M.D.1 ABSTRACT An intimate knowledge of facial nerve anatomy is critical to avoid its inadvertent injury during rhytidectomy, parotidectomy, maxillofacial fracture reduction, and almost any surgery of the head and neck. Injury to the frontal and marginal mandibular branches of the facial nerve in particular can lead to obvious clinical deficits, and areas where these nerves are particularly susceptible to injury have been designated danger zones by previous authors. Assessment of facial nerve function is not limited to its extratemporal anatomy, however, as many clinical deficits originate within its intratemporal and intracranial components. Similarly, the facial nerve cannot be considered an exclusively motor nerve given its contributions to taste, auricular sensation, sympathetic input to the middle meningeal artery, and parasympathetic innervation to the lacrimal, submandibular, and sublingual glands. The constellation of deficits resulting from facial nerve injury is correlated with its complex anatomy to help establish the level of injury, predict recovery, and guide surgical management. KEYWORDS: Extratemporal, intratemporal, facial nerve, frontal nerve, marginal mandibular nerve The anatomy of the facial nerve is among the components of the facial nerve reminds the surgeon that most complex of the cranial nerves. In his initial descrip- the facial nerve is composed not exclusively of voluntary tion of the cranial nerves, Galen described the facial motor fibers but also of parasympathetics to the lacrimal, nerve as part of a distinct facial-vestibulocochlear nerve submandibular, and sublingual glands; sensory innerva- complex.1,2 Although the anatomy of the other cranial tion to part of the external ear; and contributions to taste nerves was accurately described shortly after Galen’s at the anterior two thirds of the tongue. -
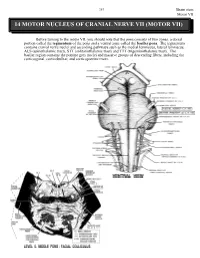
14 Motor Nucleus of Cranial Nerve Vii (Motor Vii)
263 Brain stem Motor VII 14 MOTOR NUCLEUS OF CRANIAL NERVE VII (MOTOR VII) Before turning to the motor VII, you should note that the pons consists of two zones, a dorsal portion called the tegmentum of the pons and a ventral zone called the basilar pons. The tegmentum contains cranial nerve nuclei and ascending pathways such as the medial lemniscus, lateral lemniscus, ALS (spinothalamic tract), STT (solitariothalamic tract) and TTT (trigeminothalamic tract). The basilar region contains the pontine grey nuclei and massive groups of descending fibers, including the corticospinal, corticobulbar, and corticopontine tracts. Brain stem 264 Motor VII The motor nucleus VII contains motor neurons (branchiomotor) that innervate the muscles of facial expression including the orbicularis oculi (CLOSES eyelid), the stapedius, the stylohyoid and the posterior belly of the digastric. Neurons comprising motor VII possess axons that pursue a rather circuitous route in order to exit the brain stem. Initially they pass dorsally and medially to loop over the abducens nucleus. The fibers then course ventrally and laterally to exit the brain stem. The bump in the floor of the fourth ventricle caused by the motor fibers of C.N. VII looping over the abducens nucleus is called the FACIAL COLLICULUS. A unilateral lesion interrupting the axons of C.N. VII results in the following: On the ipsilateral side, the forehead is immobile, the corner of the mouth sags, the nasolabial folds of the face are flattened, facial lines are lost, and saliva may drip from the corner of the mouth. The patient is unable to whistle or puff the cheek because the buccinator muscle is paralyzed. -

Secondary Otalgia: Referred Pain Pathways and Pathologies
Published October 22, 2020 as 10.3174/ajnr.A6808 REVIEW ARTICLE HEAD & NECK Secondary Otalgia: Referred Pain Pathways and Pathologies C.D. Norris and N.A. Koontz ABSTRACT SUMMARY: Otalgia is very common, and when the cause of ear pain is not identified on otoscopy and physical examination, cross-sectional imaging is routinely used to evaluate for potential sources of referred ear pain (secondary otalgia). Innervation of the ear structures is complex, involving multiple upper cervical, lower cranial, and peripheral nerves, which transit and innervate a large anatomic territory involving the brain, spine, skull base, aerodigestive tract, salivary glands, paranasal sinuses, face, orbits, deep spaces of the neck, skin, and viscera. Interpreting radiologists must be familiar with these neural pathways and potential sources of secondary otalgia. The purposes of this review are to detail the currently proposed mechanisms of referred ear pain, review the sa- lient neuroanatomy of the complex pathways responsible for secondary otalgia, highlight important benign and malignant etiologies of referred ear pain, and provide a structured search pattern for approaching these challenging cases on cross-sectional imaging. ABBREVIATION: CN ¼ cranial nerve; TMJ ¼ temporomandibular joint talgia is common and indiscriminate, affecting persons of all which may yield referred pain from sources outside the ear, it is Oages with nearly a 100% lifetime prevalence.1,2 Frequently, imperative that a complete history and comprehensive physical ex- the cause of otalgia can be identified with otoscopy, and when the amination be performed on patients with otalgia. pathoetiology localizes to the affected ear, it is referred to as a pri- When the cause of otalgia is not identified after an appropri- mary otalgia. -

The Fallopian Canal: a Comprehensive Review and Proposal of a New Classification
Childs Nerv Syst (2014) 30:387–395 DOI 10.1007/s00381-013-2332-0 REVIEW PAPER The fallopian canal: a comprehensive review and proposal of a new classification M. M. Mortazavi & B. Latif & K. Verma & N. Adeeb & A. Deep & C. J. Griessenauer & R. S. Tubbs & T. Fukushima Received: 1 October 2013 /Accepted: 18 November 2013 /Published online: 10 December 2013 # Springer-Verlag Berlin Heidelberg 2013 Abstract nervus intermedius, which also transmits the special sense of Introduction The facial nerve follows a complex course taste where its signals are relayed to the gustatory nucleus. The through the skull base. Understanding its anatomy is crucial parasympathetic component emerges from the superior during standard skull base approaches and resection of certain salivatory nucleus, also carried in the nervus intermedius, skull base tumors closely related to the nerve, especially, and supplies the sublingual, submandibular, nasomucosal, tumors at the cerebellopontine angle. and lacrimal glands. The motor component innervates mus- Methods Herein, we review the fallopian canal and its impli- cles of facial expression and arises from the primary facial cations in surgical approaches to the skull base. Furthermore, nucleus in the pontine tegmentum in the floor of the fourth we suggest a new classification. ventricle [1]. Conclusions Based on the anatomy and literature, we propose The facial nerve has six described segments: cisternal, that the meatal segment of the facial nerve be included as a meatal, labyrinthine, tympanic, mastoid, and extracranial [1]. component of the fallopian canal. A comprehensive knowl- The fallopian canal is a z-shaped bony channel in the temporal edge of the course of the facial nerve is important to those who bone that runs from the distal internal acoustic meatus to the treat patients with pathology of or near this cranial nerve.