Vascularisation of the Geniculate Ganglion A
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Anatomy-Nerve Tracking
INJECTABLES ANATOMY www.aestheticmed.co.uk Nerve tracking Dr Sotirios Foutsizoglou on the anatomy of the facial nerve he anatomy of the human face has received enormous attention during the last few years, as a plethora of anti- ageing procedures, both surgical and non-surgical, are being performed with increasing frequency. The success of each of those procedures is greatly dependent on Tthe sound knowledge of the underlying facial anatomy and the understanding of the age-related changes occurring in the facial skeleton, ligaments, muscles, facial fat compartments, and skin. The facial nerve is the most important motor nerve of the face as it is the sole motor supply to all the muscles of facial expression and other muscles derived from the mesenchyme in the embryonic second pharyngeal arch.1 The danger zone for facial nerve injury has been well described. Confidence when approaching the nerve and its branches comes from an understanding of its three dimensional course relative to the layered facial soft tissue and being aware of surface anatomy landmarks and measurements as will be discussed in this article. Aesthetic medicine is not static, it is ever evolving and new exciting knowledge emerges every day unmasking the relationship of the ageing process and the macroscopic and microscopic (intrinsic) age-related changes. Sound anatomical knowledge, taking into consideration the natural balance between the different facial structures and facial layers, is fundamental to understanding these changes which will subsequently help us develop more effective, natural, long-standing and most importantly, safer rejuvenating treatments and procedures. The soft tissue of the face is arranged in five layers: 1) Skin; 2) Subcutaneous fat layer; 3) Superficial musculoaponeurotic system (SMAS); 4) Areolar tissue or loose connective tissue (most clearly seen in the scalp and forehead); 5) Deep fascia formed by the periosteum of facial bones and the fascial covering of the muscles of mastication (lateral face). -

Extracranial Course of Cranial Nerves
Extracranial course of cranial nerves Oculomotor, Trochlear, Abducent, Trigeminal, Facial and Accessory nerves Dr. Heba Kalbouneh Associate Professor of Anatomy and Histology Dr. Heba Kalbouneh Brainstem Mid brain Pons Medulla Pons Inferior view Facial nerve Anatomically, the course of the facial nerve can be divided into two parts: Motor: Innervates the muscles of facial Intracranial – the course of the nerve through expression, the posterior belly of the the cranial cavity, and the cranium itself. digastric, the stylohyoid and the stapedius Extracranial – the course of the nerve outside muscles. the cranium, through the face and neck. General Sensory: A small area around the concha of the auricle, EAM Special Sensory: Provides special taste sensation to the anterior 2/3 of the tongue. Parasympathetic: Supplies many of the glands of the head and neck, including: 1- Submandibular and sublingual salivary glands (via the submandibular ganglion/ chorda tympani) 2- Nasal, palatine and pharyngeal mucous glands (via the pterygopalatine ganglion/ greater petrosal) 3- Lacrimal glands (via the pterygopalatine ganglion/ greater petrosal) Dr. Heba Kalbouneh Intracranial course The nerve arises in the pons. It begins as two roots; a large motor root, and a small sensory root The two roots travel through the internal acoustic meatus. Pons Here, they are in very close proximity to the inner ear. 7th (motor) 8th Note: The part of the facial nerve that runs between the motor root of facial and vestibulocochlear nerve is sometimes Kalbouneh known as the nervus intermedius It contains the sensory and parasympathetic Heba fibers of the facial nerve Dr. Dr. Still within the temporal bone, the roots leave the internal acoustic meatus, and enter into the facial canal. -
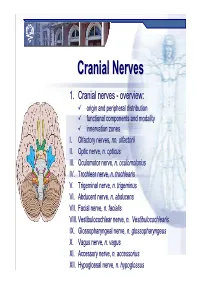
Cranial Nervesnerves
CranialCranial NervesNerves 1. Cranial nerves - overview: origin and peripheral distribution functional components and modality innervation zones I. Olfactory nerves, nn. olfactorii II. Optic nerve, n. opticus III. Oculomotor nerve, n. oculomotorius IV. Trochlear nerve, n. trochlearis V. Trigeminal nerve, n. trigeminus VI. Abducent nerve, n. abducens VII. Facial nerve, n. facialis VIII. Vestibulocochlear nerve, n. Vestibulocochlearis IX. Glossopharyngeal nerve, n. glossopharyngeus X. Vagus nerve, n. vagus XI. Accessory nerve, n. accessorius XII. Hypoglossal nerve, n. hypoglossus Cranial nerves CranialCranial nervesnerves 0. N. terminalis I. N. olfactorius II. N. opticus III. N. oculomotorius IV. N. trochlearis V. N. trigeminus VI. N. abducens VII. N. facialis VIII. N. vestibulocochlearis IX. N. glossopharyngeus X. N. vagus XI. N. accessorius XII. N. hypoglossus Prof. Dr. Nikolai Lazarov 2 Cranial nerves FunctionalFunctional classificationclassification purely sensory (afferent ): n. olfactorius n. opticus n. vestibulocochlearis purely motor (efferent): n. oculomotorius n. trochlearis n. abducens n. accessorius n. hypoglossus mixed (sensory&motor ): n. trigeminus n. facialis n. glossopharyngeus n. vagus autonomic (parasympathetic( ): n. oculomotorius n. facialis n. glossopharyngeus n. vagus Prof. Dr. Nikolai Lazarov 3 Cranial nerves AnatomicAnatomic relationshipsrelationships Location within the brainstem: ventrally: n. olfactorius n. opticus n. oculomotorius n. abducens n. hypoglossus laterally: n. trigeminus -

Morphometry and Morphology of Foramen Petrosum in Indian Population
Basic Sciences of Medicine 2020, 9(1): 8-9 DOI: 10.5923/j.medicine.20200901.02 Morphometry and Morphology of Foramen Petrosum in Indian Population Rajani Singh1,*, Nand Kishore Gupta1, Raj Kumar2 1Department of Anatomy, Uttar Pradesh University of Medical Sciences Saifai 206130 Etawah UP India 2Department of Neurosugery Uttar Pradesh University of Medical Sciences Saifai 206130 Etawah UP India Abstract Greater wing of sphenoid contains three constant foramina, Foramen ovale, foramen rotundum and foramen spinosum. The presence of foramen Vesalius and foramen petrosum are inconsistent. Normally foramen ovale transmits mandibular nerve, accessory meningeal artery, lesser petrosal nerve and emissary vein. When foramen petrosum is present, lesser petrosal nerve passes through petrosal foramen instead of foramen ovale. Lesser petrosal nerve distribute postganglionic fibers from otic ganglion to parotid gland. In absence of knowledge of petrosal foramen transmitting lesser petrosal nerve, the clinician may damage the nerve during skull base surgery creating complications like hyperemia of face and profuse salivation from the parotid gland (following atropine administration), lacrimation (crocodile tears syndrome) and mucus nasal secretion. Considering clinical implications associated with petrosal foramen, the study was carried out. The aim of the study is to determine the prevalence of petrosal foramen in Indian Population and to bring out associated clinical significance. The study was conducted in the department of Anatomy UPUMS Saifai Etawah Indian. 30 half skulls were observed for the presence of petrosal foramina and morphometry was also done. Literature search was carried out, our findings were compared with previous work and associated clinical implications were bought out. Keywords Petrosal foramen, Lesser petrosal nerve, Foramen ovale patients. -

Brain Structure and Function Related to Headache
Review Cephalalgia 0(0) 1–26 ! International Headache Society 2018 Brain structure and function related Reprints and permissions: sagepub.co.uk/journalsPermissions.nav to headache: Brainstem structure and DOI: 10.1177/0333102418784698 function in headache journals.sagepub.com/home/cep Marta Vila-Pueyo1 , Jan Hoffmann2 , Marcela Romero-Reyes3 and Simon Akerman3 Abstract Objective: To review and discuss the literature relevant to the role of brainstem structure and function in headache. Background: Primary headache disorders, such as migraine and cluster headache, are considered disorders of the brain. As well as head-related pain, these headache disorders are also associated with other neurological symptoms, such as those related to sensory, homeostatic, autonomic, cognitive and affective processing that can all occur before, during or even after headache has ceased. Many imaging studies demonstrate activation in brainstem areas that appear specifically associated with headache disorders, especially migraine, which may be related to the mechanisms of many of these symptoms. This is further supported by preclinical studies, which demonstrate that modulation of specific brainstem nuclei alters sensory processing relevant to these symptoms, including headache, cranial autonomic responses and homeostatic mechanisms. Review focus: This review will specifically focus on the role of brainstem structures relevant to primary headaches, including medullary, pontine, and midbrain, and describe their functional role and how they relate to mechanisms -

Surgical Treatment of Bronchial Asthma by Resection of the Laryngeal Nerve
Surgical treatment of bronchial asthma by resection of the laryngeal nerve Ubaidullo Kurbon, Hamza Dodariyon, Abdumalik Davlatov, Sitora Janobilova, Amu Therwath, Massoud Mirshahi To cite this version: Ubaidullo Kurbon, Hamza Dodariyon, Abdumalik Davlatov, Sitora Janobilova, Amu Therwath, et al.. Surgical treatment of bronchial asthma by resection of the laryngeal nerve. BMC Surgery, BioMed Central, 2015, 15 (1), pp.109. 10.1186/s12893-015-0093-2. inserm-01264486 HAL Id: inserm-01264486 https://www.hal.inserm.fr/inserm-01264486 Submitted on 29 Jan 2016 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Kurbon et al. BMC Surgery (2015) 15:109 DOI 10.1186/s12893-015-0093-2 TECHNICAL ADVANCE Open Access Surgical treatment of bronchial asthma by resection of the laryngeal nerve Ubaidullo Kurbon1, Hamza Dodariyon1, Abdumalik Davlatov1, Sitora Janobilova1, Amu Therwath2 and Massoud Mirshahi1,2* Abstract Background: Management of asthma in chronically affected patients is a serious health problem. Our aim was to show that surgical treatment of chronic bronchial asthma by unilateral resection of the internal branch of the superior laryngeal nerve (ib-SLN) is an adequateand lasting remedial response. Patients and methods: In a retrospective study, 41 (26 male and 15 female) patients with bronchial chronic asthma were treated surgically during the period between 2005 and 2013. -

Greater Superficial Petrosal Nerve Schwannoma: 3 Case Reports and Review of Literature
Send Orders for Reprints to [email protected] The Open Neurosurgery Journal, 2015, 7, 1-5 1 Open Access Greater Superficial Petrosal Nerve Schwannoma: 3 Case Reports and Review of Literature Khursheed A. Ansari*,1, Girish Menon2, Mathew Abraham2 and Suresh Nair2 1Department of Neurosurgery, Wockhardt Hospitals, The Umrao Institute of Medical Sciences and Research, Mira road, North Bombay, India-401107 2Department of Neurosurgery, Sree Chitra Tirunal Institute for Medical sciences and technology, Trivandrum, Kerala, India-695011 Abstract: Greater superficial petrosal nerve (GSPN) schwannomas are very rare tumors. There are only 15 reports, described so far. In this study the authors discuss the clinical presentation, radiological features, surgical approach, post- operative outcome and follow up of 3 cases of this rare schwannoma of the middle fossa, possibly originating from GSPN together with the review of existing literature. The presenting features were facial paresis in all cases, xerophthalmia in case 1, and tinnitus in case 2. Imaging studies showed a sub-temporal extradural mass on the anterosuperior aspect of the petrous bone in all cases. All tumors were removed through a subtemporal extradural microsurgical approach. Improvement of facial weakness and xerophthalmia as an end result is characteristic post-operative finding. Keywords: Facial nerve, greater superficial petrosal nerve, middle fossa, schwannoma, xerophthalmia. INTRODUCTION of the schwannoma. The tumor was eroding the anterosuperior aspect of petrous bone and was abutting the Schwannomas of middle cranial fossa commonly arise geniculate part of VIIth nerve. Postoperatively his facial from the trigeminal nerve but may arise from facial nerve or palsy improved to HB grad III with xerophthamia. -

Clinical Anatomy of Greater Petrosal Nerve and Its Surgical Importance
[Downloaded free from http://www.indianjotol.org on Monday, August 18, 2014, IP: 218.241.189.21] || Click here to download free Android application for this journal ORIGINAL ARTICLE Clinical anatomy of greater petrosal nerve and its surgical importance Prashant E Natekar, Fatima M De Souza Department of Anatomy, Goa Medical College, Bambolim, Goa, India Background: Surgical approach towards greater petrosal nerve has to be done with caution as many surgeons ABSTRACT are unfamiliar with the anatomy of the facial nerve. The anatomical landmarks selected must be reliable and above all easy to identify for identification of the greater petrosal nerve so as to avoid injury to the structures in the middle cranial fossa. Observation and Results: The present study is carried out on 100 temporal bones by examining the following measurements of the right and the left sides a) length of the hiatus for grater petrosal superficial nerve b) distance from superior petrosal sinus c) distance from lateral margin of middle cranial fossa d) arcuate eminence and e) distance from exit to the foramen ovale. Conclusion: The anatomical landmarks selected must be reliable and above all easy to identify. Bony structures are more suitable than soft tissue or cartilaginous landmarks because of their rigid and reliable location. These anatomical landmarks will definitely help the surgeon while performing vidian nerve neurectomy and also the anatomical relationship of the facial nerve in temporal bone. The middle fossa approach involves a temporal craniotomy in cases of perineural spread of adenoid cystic carcinomas hence these anatomical landmarks will serve as useful guide for the surgeons and radiologists. -

Parasympathetic Nucleus of Facial Nerve
Neurology of Lacrimation Michael Davidson Professor, Ophthalmology Diplomate, American College of Veterinary Ophthalmologists Department of Clinical Sciences College of Veterinary Medicine North Carolina State University Raleigh, North Carolina, USA Trigeminal Nerve and Lacrimation Sensory afferent from lacrimal gland, adnexa, eye – through ophthalmic division and first branch of maxillary division (zygomatic n.) maxillary branch To trigeminal ganglion ophthalmic branch – adjacent to petrous temporal bone lateral to mandibular branch cavernous sinus near middle ear trigeminal ganglion Terminal branches of trigeminal n. trigeminal nerve distributes sympathetic and parasympathetic efferent to lacrimal gland and face Trigeminal-Lacrimal Reflex Afferent arm = CN V, ophthalmic division ⇒trigeminal ganglion ⇒ principle CN V nuclei Efferent arm = parasympathetic efferent to lacrimal gland(s) Elicits reflex tearing Parasympathetic efferent also supplies basal stimuli for lacrimation www.slideplayer.com Parasympathetic Efferent to Lacrimal Gland CN VII parasympathetic nuclei ⇒ greater petrosal nerve (petrous temporal bone) ⇒ nerve of pterygoid canal (with post ganglionic sympathetics) ⇒ synapse in pterygopalatine ganglion (ventral periorbital region, near apex of orbit) ⇒ zygomatic nerve (CN V, maxillary division)* ⇒ zygomaticotemporal nerve ⇒ acinar cells of lacrimal gland** *some texts state postganglionic parasympathetic fibers also in lacrimal nerve (ophthalmic division CNV) **postganglionic parasympathetic innervation to nictitans -

Clinical Anatomy of the Cranial Nerves Clinical Anatomy of the Cranial Nerves
Clinical Anatomy of the Cranial Nerves Clinical Anatomy of the Cranial Nerves Paul Rea AMSTERDAM • BOSTON • HEIDELBERG • LONDON NEW YORK • OXFORD • PARIS • SAN DIEGO SAN FRANCISCO • SINGAPORE • SYDNEY • TOKYO Academic Press is an imprint of Elsevier Academic Press is an imprint of Elsevier 32 Jamestown Road, London NW1 7BY, UK The Boulevard, Langford Lane, Kidlington, Oxford OX5 1GB, UK Radarweg 29, PO Box 211, 1000 AE Amsterdam, The Netherlands 225 Wyman Street, Waltham, MA 02451, USA 525 B Street, Suite 1800, San Diego, CA 92101-4495, USA First published 2014 Copyright r 2014 Elsevier Inc. All rights reserved. No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, or any information storage and retrieval system, without permission in writing from the publisher. Details on how to seek permission, further information about the Publisher’s permissions policies and our arrangement with organizations such as the Copyright Clearance Center and the Copyright Licensing Agency, can be found at our website: www.elsevier.com/permissions. This book and the individual contributions contained in it are protected under copyright by the Publisher (other than as may be noted herein). Notices Knowledge and best practice in this field are constantly changing. As new research and experience broaden our understanding, changes in research methods, professional practices, or medical treatment may become necessary. Practitioners and researchers must always rely on their own experience and knowledge in evaluating and using any information, methods, compounds, or experiments described herein. In using such information or methods they should be mindful of their own safety and the safety of others, including parties for whom they have a professional responsibility. -

In Vivo Neurophysiological Recordings From
University of Nebraska at Omaha DigitalCommons@UNO Psychology Faculty Publications Department of Psychology 2005 In vivo neurophysiological recordings from geniculate ganglia: taste response properties of individual greater superficial petrosal and chorda tympani neurones Suzanne I. Sollars University of Nebraska at Omaha, [email protected] David L. Hill University of Virginia Follow this and additional works at: https://digitalcommons.unomaha.edu/psychfacpub Part of the Psychology Commons Recommended Citation Sollars, Suzanne I. and Hill, David L., "In vivo neurophysiological recordings from geniculate ganglia: taste response properties of individual greater superficial petrosal and chorda tympani neurones" (2005). Psychology Faculty Publications. 227. https://digitalcommons.unomaha.edu/psychfacpub/227 This Article is brought to you for free and open access by the Department of Psychology at DigitalCommons@UNO. It has been accepted for inclusion in Psychology Faculty Publications by an authorized administrator of DigitalCommons@UNO. For more information, please contact [email protected]. tjp˙828 TJP-xml.cls March14, 2005 20:32 JPhysiol 000.0 (2005) pp 1–17 1 In vivo neurophysiological recordings from geniculate ganglia: taste response properties of individual greater superficial petrosal and chorda tympani neurones Suzanne I. Sollars1 and David L. Hill2 1Department of Psychology, University of Nebraska Omaha, Omaha, NE 68182, USA 2Department of Psychology, University of Virginia, Charlottesville, VA 22904, USA Coding of gustatory information is complex and unique among sensory systems; information is received by multiple receptor populations located throughout the oral cavity and carried to a single central relay by four separate nerves. The geniculate ganglion is the location of the somata of two of these nerves, the greater superficial petrosal (GSP) and the chorda tympani (CT). -

Neural Control of Swallowing
AG-2018-48 REVIEW dx.doi.org/10.1590/S0004-2803.201800000-45 Neural control of swallowing Milton Melciades Barbosa COSTA Received 11/4/2018 Accepted 9/5/2018 ABSTRACT – Background – Swallowing is a motor process with several discordances and a very difficult neurophysiological study. Maybe that is the reason for the scarcity of papers about it. Objective – It is to describe the chewing neural control and oral bolus qualification. A review the cranial nerves involved with swallowing and their relationship with the brainstem, cerebellum, base nuclei and cortex was made. Methods – From the reviewed literature including personal researches and new observations, a consistent and necessary revision of concepts was made, not rarely conflicting. Re- sults and Conclusion – Five different possibilities of the swallowing oral phase are described: nutritional voluntary, primary cortical, semiautomatic, subsequent gulps, and spontaneous. In relation to the neural control of the swallowing pharyngeal phase, the stimulus that triggers the pharyngeal phase is not the pharyngeal contact produced by the bolus passage, but the pharyngeal pressure distension, with or without contents. In nutritional swallowing, food and pressure are transferred, but in the primary cortical oral phase, only pressure is transferred, and the pharyngeal response is similar. The pharyngeal phase incorporates, as its functional part, the oral phase dynamics already in course. The pharyngeal phase starts by action of the pharyngeal plexus, composed of the glossopharyngeal (IX), vagus (X) and accessory (XI) nerves, with involvement of the trigeminal (V), facial (VII), glossopharyngeal (IX) and the hypoglossal (XII) nerves. The cervical plexus (C1, C2) and the hypoglossal nerve on each side form the ansa cervicalis, from where a pathway of cervical origin goes to the geniohyoid muscle, which acts in the elevation of the hyoid-laryngeal complex.