Changes in L4/5 Intervertebral Foramen Bony Morphology With
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Anatomy of the Spine
12 Anatomy of the Spine Overview The spine is made of 33 individual bones stacked one on top of the other. Ligaments and muscles connect the bones together and keep them aligned. The spinal column provides the main support for your body, allowing you to stand upright, bend, and twist. Protected deep inside the bones, the spinal cord connects your body to the brain, allowing movement of your arms and legs. Strong muscles and bones, flexible tendons and ligaments, and sensitive nerves contribute to a healthy spine. Keeping your spine healthy is vital if you want to live an active life without back pain. Spinal curves When viewed from the side, an adult spine has a natural S-shaped curve. The neck (cervical) and low back (lumbar) regions have a slight concave curve, and the thoracic and sacral regions have a gentle convex curve (Fig. 1). The curves work like a coiled spring to absorb shock, maintain balance, and allow range of motion throughout the spinal column. The muscles and correct posture maintain the natural spinal curves. Good posture involves training your body to stand, walk, sit, and lie so that the least amount of strain is placed on the spine during movement or weight-bearing activities. Excess body weight, weak muscles, and other forces can pull at the spine’s alignment: • An abnormal curve of the lumbar spine is lordosis, also called sway back. • An abnormal curve of the thoracic spine is Figure 1. (left) The spine has three natural curves that form kyphosis, also called hunchback. an S-shape; strong muscles keep our spine in alignment. -

Chapter 02: Netter's Clinical Anatomy, 2Nd Edition
Hansen: Netter's Clinical Anatomy, 2nd Edition - with Online Access 2 BACK 1. INTRODUCTION 4. MUSCLES OF THE BACK REVIEW QUESTIONS 2. SURFACE ANATOMY 5. SPINAL CORD 3. VERTEBRAL COLUMN 6. EMBRYOLOGY FINAL 1. INTRODUCTION ELSEVIERl VertebraeNOT prominens: the spinous process of the C7- vertebra, usually the most prominent The back forms the axis (central line) of the human process in the midline at the posterior base of body and consists of the vertebral column, spinal cord, the neck supporting muscles, and associated tissues (skin, OFcon- l Scapula: part of the pectoral girdle that sup- nective tissues, vasculature, and nerves). A hallmark of ports the upper limb; note its spine, inferior human anatomy is the concept of “segmentation,” and angle, and medial border the back is a prime example. Segmentation and bilat l Iliac crests: felt best when you place your eral symmetry of the back will be obvious as you hands “on your hips”; an imaginary horizontal study the vertebral column, the distribution of the line connecting the crests passes through the spinal nerves, the muscles of th back, and its vascular spinous process of the L4 vertebra and the supply. intervertebral disc of L4-L5, a useful landmark Functionally, the back is involved in three primary for a lumbar puncture or epidural block tasks: l Posterior superior iliac spines: an imaginary CONTENThorizontal line connecting these two points l Support: the vertebral column forms the axis of passes through the spinous process of S2 (second the body and is critical for our upright posture sacral segment) (standing or si ting), as a support for our head, as an PROPERTYattachment point and brace for move- 3. -

Bones of the Trunk
BONES OF THE TRUNK Andrea Heinzlmann Veterinary University Department of Anatomy and Histology 16th September 2019 VERTEBRAL COLUMN (COLUMNA VERTEBRALIS) • the vertebral column composed of the vertebrae • the vertebrae form a horizontal chain https://hu.pinterest.com/pin/159877855502035893/ VERTEBRAL COLUMN (COLUMNA VERTEBRALIS) along the vertebral column three major curvatures are recognized: 1. the DORSAL CONVEX CURVATURE – between the head and the neck 2. the DORSAL CONCAVE CURVATURE – between the neck and the chest 3. the DORSAL CONVEX CURVATURE – between the thorax and the lumbar region - in carnivores (Ca) there is an additional DORSAL CONVEXITY in the sacral region https://hu.pinterest.com/pin/159877855502035893/ VERTEBRAL COLUMN (COLUMNA VERTEBRALIS) - corresponding to the regions of the body, we distinguish: 1. CERVICAL VERTEBRAE 2. THORACIC VERTEBRAE 3. LUMBAR VERTEBRAE 4. SACRAL VERTEBRAE 5. CAUDAL (COCCYGEAL) VERTEBRAE https://www.ufaw.org.uk/dogs/french-bulldog-hemivertebrae https://rogueshock.com/know-your-horse-in-9-ways/5/ BUILD OF THE VERTEBRAE each vertebrae presents: 1. BODY (CORPUS VERTEBRAE) 2. ARCH (ARCUS VERTEBRAE) 3. PROCESSES corpus Vertebra thoracica (Th13) , Ca. THE VERTEBRAL BODY (CORPUS VERTEBRAE) - the ventral portion of the vertebra ITS PARTS: 1. EXTREMITAS CRANIALIS (seu CAPUT VERTEBRAE) – convex 2. EXTREMITAS CAUDALIS (seu FOSSA VERTEBRAE) - concave Th13, Ca. THE VERTEBRAL BODY (CORPUS VERTEBRAE) 3. VENTRAL SURFACE of the body has a: - ventral crest (CRISTA VENTRALIS) 4. DORSAL SURFACE of the body carries : - the vertebral arch (ARCUS VERTEBRAE) Th13, Ca., lateral aspect Arcus vertebrae corpus Vertebra thoracica (Th13) , Ca., caudal aspect THE VERTEBRAL BODY (CORPUS VERTEBRAE) 6. VERTEBRAL ARCH (ARCUS VERTEBRAE) compraisis: a) a ventral PEDICULUS ARCUS VERTEBRAE b) a dorsal LAMINA ARCUS VERTEBRAE C7, Ca. -

Radiographic Anatomy of the Intervertebral Cervical and Lumbar Foramina 691
Diagnostic and Interventional Imaging (2012) 93, 690—697 View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector CONTINUING EDUCATION PROGRAM: FOCUS. Radiographic anatomy of the intervertebral cervical and lumbar foramina (vessels and variants) ∗ X. Demondion , G. Lefebvre, O. Fisch, L. Vandenbussche, J. Cepparo, V. Balbi Service de radiologie musculosquelettique, CCIAL, laboratoire d’anatomie, faculté de médecine de Lille, hôpital Roger-Salengro, CHRU de Lille, rue Émile-Laine, 59037 Lille, France KEYWORDS Abstract The intervertebral foramen is an orifice located between any two adjacent verte- Spine; brae that allows communication between the spinal (or vertebral) canal and the extraspinal Spinal cord; region. Although the intervertebral foramina serve as the path traveled by spinal nerve roots, Vessels vascular structures, including some that play a role in vascularization of the spinal cord, take the same path. Knowledge of this vascularization and of the origin of the arteries feeding it is essential to all radiologists performing interventional procedures. The objective of this review is to survey the anatomy of the intervertebral foramina in the cervical and lumbar spines and of spinal cord vascularization. © 2012 Éditions françaises de radiologie. Published by Elsevier Masson SAS. All rights reserved. The intervertebral foramen is an orifice located between any two adjacent vertebrae that allows communication between the spinal (or vertebral) canal and the extraspinal region. Although the intervertebral foramina serve as the path traveled by spinal nerve roots, vascular structures, including some that play a role in vascularization of the spinal cord, take the same path. Knowledge of this vascularization and of the source of the arteries feeding the spinal cord is therefore essential to all radiologists performing interventional procedures in view of the iatrogenic risks they present. -

Spinal Nerve Position and Morphometric Analysis with Silicon Molds in the Cadaveric Lumbar Intervertebral Foramen
대한체질인류학회지 제 31 권 제 4 호 Korean J Phys Anthropol Vol. 31, No. 4 (2018) pp. 151~158 https://doi.org/10.11637/kjpa.2018.31.4.151 Original Article Spinal Nerve Position and Morphometric Analysis with Silicon Molds in the Cadaveric Lumbar Intervertebral Foramen Soonwook Kwon Department of Anatomy, School of Medicine, Catholic University of Daegu (Received 4 December 2018, revised 14 December 2018, accepted 17 December 2018) Abstract : The intervertebral foramen is formed by two adjacent vertebrae and an intervertebral disc. Previous studies examining the foramen have been performed using various methods. The author obtained characteristics of the intervertebral foramen based on silicon mold. The author used 18 cadavers and dissected the lumbar intervertebral foramen. First, positional levels of the spinal nerve in the intervertebral foramen were measured. Second, after being removed all tissues covering the intervertebral, bony foramen was filled with melted silicon to mold the cross section. Subsequently, the solidified silicon mold was removed and stamped on a paper. The paper was scanned and analyzed area, perimeter, height and width of the intervertebral foramen on a computer. Area (average, 9.43 mm2) and perimeter (average, 48.02 mm) did not show any statistical significant pattern for any lumbar vertebral levels. However, the height and width significantly differed at the fifth lumbar vertebra, which had the shortest height (the fifth, 13.00 mm; average, 15.78 mm) and longest width (the fifth, 8.61 mm; average, 7.87 mm), although there were similar patterns in case of area and perimeter of the first to fourth lumbar vertebra. -

Clinical Anatomy and Significance of the Lumbar
j o u r n a l o f t h e a n a t o m i c a l s o c i e t y o f i n d i a 6 4 ( 2 0 1 5 ) 1 6 6 – 1 7 3 Available online at www.sciencedirect.com ScienceDirect journal homepage: www.elsevier.com/locate/jasi Review Article Clinical anatomy and significance of the lumbar intervertebral foramen: A review a b,c, Grigorios Gkasdaris , Stylianos Kapetanakis * a Department of Anatomy, Medical School, Democritus University of Thrace, Alexandroupolis 68100, Greece b Orthopaedic Surgeon-Spine Surgeon, Lecturer of Anatomy, Department of Anatomy, Medical School, Democritus University of Thrace, Alexandroupolis 68100, Greece c European Interbalkan Medical Center, Thessaloniki 57001, Greece a r t i c l e i n f o a b s t r a c t Article history: The objective of this review is to summarize the knowledge about the anatomy of the lumbar Received 8 August 2015 intervertebral foramen (IVF) as an anatomic entity. The intervertebral or neural foramen is Accepted 26 October 2015 an orifice located between any two adjacent vertebrae that allows communication between Available online 23 November 2015 the spinal canal and the extraspinal region. We describe the osseous structure of the lumbar foramen, the adjacent ligaments and its components including the arteries and veins Keywords: passing through or neighboring it, and the spinal nerves and roots. Although lumbar spine structures are familiar to spinal surgeons and many procedures are performed in the area of Lumbar intervertebral foramen Anatomy the lumbar IVF, yet surprisingly little is known about the precise anatomy of the foramen Clinical and the triangular working zone of it. -
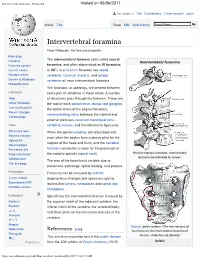
Intervertebral Foramina - Wikipedia Visited on 06/06/2017
Intervertebral foramina - Wikipedia Visited on 06/06/2017 Not logged in Talk Contributions Create account Log in Article Talk Read Edit View history Intervertebral foramina From Wikipedia, the free encyclopedia Main page The intervertebral foramen (also called neural Contents Intervertebral foramina Featured content foramina, and often abbreviated as IV foramina Current events or IVF), is a foramen between two spinal Random article vertebrae. Cervical, thoracic, and lumbar Donate to Wikipedia vertebrae all have intervertebral foramina. Wikipedia store The foramina, or openings, are present between Interaction every pair of vertebrae in these areas. A number Help of structures pass through the foramen. These are About Wikipedia the root of each spinal nerve, dorsal root ganglion, Community portal the spinal artery of the segmental artery, Recent changes communicating veins between the internal and Contact page external plexuses, recurrent meningeal (sinu- Tools vertebral) nerves, and transforaminal ligaments. What links here When the spinal vertebrae are articulated with Related changes each other the bodies form a strong pillar for the Upload file support of the head and trunk, and the vertebral Special pages Permanent link foramen constitutes a canal for the protection of Page information the medulla spinalis (spinal cord). Peculiar thoracic vertebrae. Intervertebral foramina are indicated by arrows. Wikidata item The size of the foramina is variable due to Cite this page placement, pathology, spinal loading, and posture. Print/export Foramina can be occluded by arthritic Create a book degenerative changes and space-occupying Download as PDF lesions like tumors, metastases and spinal disc Printable version herniations. Languages Specifically the intervertebral foramen is bound by Deutsch the superior notch of the adjacent vertebra, the Español inferior notch of the vertebra, the vertebral body, and facet joints on the transverse process of the فارسی Français vertebra. -

Anatomical Studies on the Vertebral Column, Sternum and Ribs of Bar Headed Goose (Anser Indicus)
Veterinary Practitioner Vol. 18 No. 2 December 2017 ANATOMICAL STUDIES ON THE VERTEBRAL COLUMN, STERNUM AND RIBS OF BAR HEADED GOOSE (ANSER INDICUS) P.J. Doley1, Shalini Suri 2 and K. Sarma3 Division of Veterinary Anatomy, Faculty of Veterinary Sciences and Animal Husbandry Sher-e-Kashmir University of Agricultural Sciences and Technology- Jammu, R.S.Pura-181 102, Jammu and Kashmir, India ABSTRACT Revised Received on: 22.05.2017 Accepted on: 28.08.2017 The vertebral column of bar headed goose consisted of 17 cervical vertebrae, 9 thoracic vertebrae, a synsacrum and 5 coccygeal vertebrae. The first two cervical vertebrae were atypical and the remaining 15 were typical showing a large anterior extremity. The bodies of the first 6 adjacent thoracic vertebrae and the free thoracic vertebrae articulated with each other by means of a saddle joint while the last three fused with the synsacrum. The anterior aspect of the bodies of the cervical and thoracic vertebrae showed a transversely concave and dorsoventrally convex articular facet whereas the caudal articular surface showed the opposite curva- ture. Cranio-caudally the synsacrum showed synsacrothoracic vertebrae with well developed dorsal spines and transverse pro- cesses, synsacrolumbar vertebrae, primary sacral vertebrae and synsacrocaudal vertebrae. The sternum of the bar head goose was in the form of quadrilateral bony plate. There were nine pairs of ribs in bar headed goose of which the first pair was floating. Key words: Bar headed goose, vertebral column, synsacrum, ribs and sternum Introduction showed an articular facet for the body of the axis. The bar headed goose (Anser indicus) is known locally The anterior surface of the body of the axis gave origin to a as Rajhans in India. -

Anatomy of VERTEBRAL COLUMN
AnatomyAnatomy ofof VERTEBRALVERTEBRAL COLUMNCOLUMN By Hermizan Halihanafiah 1 The vertebral column, also called the spine, spinal column or backbone. Composed of a series of bones called vertebrae (singular is vertebra). About 71 cm (28in): adult male. About 61 cm (24in): adult female. 2 Total number of vertebrae during early development is 33. As a child grows, several vertebrae in the sacral and coccygeal regions fuse. Adults have 26 vertebrae. *Sacrum and coccyx bones become fused. 7 cervical vertebrae (C1 - C7 ) 12 thoracic vertebrae (T1 –T12) 5 lumbar vertebrae (L1 – L5) 1 sacrum ( 5 fused ) 1 coccyx ( 4 fused ) *The sacrum and coccyx do not have number. The cervical, thoracic and lumbar vertebrae movable Sacrum and coccyx immovable Between adjacent vertebrae from the second cervical vertebra to the sacrum are intervertebral disc (inter = between). 5 6 GeneralGeneral StructureStructure ofof VertebraeVertebrae 1. Cervical vertebrae (C1-C7) • Formed framework of neck region • Support skull • Small in size • Presense foramen in each transverse process 2. Thoracic vertebrae (T1-T12) • Formed posterior part of thoracic cage • Articulates with associated ribs 7 3. Lumbar Vertebrae (L1-L5) • Formed skeletal support for posterior abdominal wall • Characterized by large in size 4. Sacrum Vertebrae • Fusion of 5 sacral bones • Immovable (synostosis) • Articulates with L5 at lumbosacral joint • Articulates laterally with pelvic bone at sacroiliac joint. • Formed posterior wall of lower abdominal and pelvic cavity 5. Coccyx • Fusion of 4 coccyx bones • Immovable (synostosis) • Formed part of pelvic cavity 8 1. Supports the head. 2. Help maintain balance in the upright position. 3. Enclose and protect the spinal cord. -

Bones and Joints of Axial Skeleton
Unit 1: Bones and joints of axial skeleton GENERAL OBJECTIVES: - recognize, name and correctly position vertebral bony elements - explain how the structure of vertebral column serves its function - understand vertebral movements - name and describe all vertebral joints - name and describe elements of the thoracic cage SPECIFIC OBJECTIVES: Identify major bony features of a typical CERVICAL, THORACIC & LUMBAR vertebrae BODY VERTEBRAL ARCH (pedicles, laminae) PROCESSES (transverse, spinous, articular) JOINT SURFACES - intervertebral disc - for (zyg)apophyseal or "facet" joints - for ribs (thoracic vertebra) Cervical vertebrae Identify the bony features on: typical cervical vertebrae (C3 - C6), vertebrae prominens (C7), atlas (Cl) & axis (C2) Deduce (from the articular facets) the movements of the: (i) cervical spine (ii) atlanto-axial joint and (iii) atlanto-occipital joint Indicate the bony attachments of major ligaments maintaining joint stability (while allowing movements). Identify the boundaries of the intervertebral foraminae. Indicate (i) the particular spinal nerve which emerges from each intervertebral foramen (noting that there are 8 cervical spinal nerves yet only 7 cervical vertebrae) (ii) where the vertebral vessels travel (including the relationship to the atlas). Typical Cervical Joints of Bodies: Intervertebral Discs Uncovertebral Joints of Vertebral Arches: Zygaphophyseal Joints Ligaments of Cervical Joints Movements of the Cervical Spine (Neck): Flexion/Extension Lateral Flexion Atlanto-Occipital & Atlanto-Axial Joints Ligaments of Joints of Atlas Movements at the Joints of the Atlas (i.e. below & above) Rotation (at Atlanto-Axial) of the Neck Flexion/Extension (at Atlanto-Occipital) of the Head Thoracic and lumbar vertebrae Compare and contrast the following groups of thoracic and lumbar vertebrae: Upper Thoracic, Middle Thoracic, Lower Thoracic, Upper Lumbar & Lower Lumbar Define the boundaries of an intervertebral foramen (noting the intervertebral disc anteriorly and the zygapophyseal joints posteriorly). -

Know Left/Right for All Bones Except Vertebrae, Sternum, and Patella Upper Extremity Clavicle Sternal End Acromial End Conoid Tu
Know left/right for all bones except vertebrae, sternum, and patella Upper Extremity Lower Extremity Clavicle Os coxa sternal end obturator foramen acromial end acetabulum conoid tubercle Ilium Scapula anterior superior iliac spine spine anterior inferior iliac spine acromion process posterior superior iliac spine coracoid process posterior inferior iliac spine subscapular fossa iliac crest supraspinous/infraspinous fossa iliac fossa vertebral (medial) border auricular (articular)surface for sacro- glenoid cavity (fossa) iliac joint suprascapular notch greater sciatic notch inferior angle superior angle Pubis pubic crest and pubic tubercle Humerus superior/inferior pubic rami head area of pubic symphysis anatomical/surgical neck greater/lesser tubercle Ischium intertubercular (biciptal) groove ischial tuberosity deltoid tuberosity ischial spine radial (spiral) groove lesser sciatic notch olecranon fossa ischial ramus trochlea capitulum Femur coronoid fossa head medial/lateral epicondyle anatomical/surgical neck greater/lesser trochanter Radius medial/lateral condyle head/neck medial/lateral epicondyle ulnar notch adductor tubercle radial tuberosity linea aspera interosseous ridge (border) fovea capitis styloid process intertrochanteric line intertrochanteric crest Ulna head/neck Patella styloid process olecranon process Tibia coronoid process tibial tuberosity ulnar tuberosity medial malleolus trochlear notch interosseous ridge (border) radial notch medial/lateral condyle interosseous ridge (border) intercondylar eminence Wrist/Hand Fibula -
![[Half-Title to Come] 10752-01 Ch01redo.Qxd 9/24/07 3:07 PM Page 2](https://docslib.b-cdn.net/cover/8984/half-title-to-come-10752-01-ch01redo-qxd-9-24-07-3-07-pm-page-2-6708984.webp)
[Half-Title to Come] 10752-01 Ch01redo.Qxd 9/24/07 3:07 PM Page 2
10752-01_CH01redo.qxd 9/24/07 3:07 PM Page 1 [Half-Title to come] 10752-01_CH01redo.qxd 9/24/07 3:07 PM Page 2 Lippincott Williams & Wilkins atlas of ANATOMY THE BACK CHAPTER 1 Plate 1-01 Palpable Structures of the Back . 5 Plate 1-02 Vertebral Column, Lateral View . 6 Plate 1-03 Cervical Vertebrae . 7 Plate 1-04 Articulated Cervical Vertebrae . 8 Plate 1-05 Thoracic and Lumbar Vertebrae . 9 Plate 1-06 Articulated Thoracic Vertebrae . 10 Plate 1-07 Articulated Lumbar Vertebrae . 11 Plate 1-08 Sacrum and Coccyx . 12 Plate 1-09 Ligaments of the Cervical Vertebrae . 13 Plate 1-10 Ligaments of the Thoracic Vertebrae . 14 Plate 1-11 Ligaments of the Lumbar Vertebrae and Sacrum . 15 Plate 1-12 Cutaneous Innervation of the Back . 16 Plate 1-13 Superficial Muscles of the Back . 17 Plate 1-14 Deep Back Muscles, Superficial Dissection . 18 Plate 1-15 Deep Back Muscles, Deeper Dissection . 19 Plate 1-16 Suboccipital Region. 20 Plate 1-17 Pattern of a Typical Spinal Nerve . 21 Plate 1-18 Spinal Cord, Posterior View . 22 Plate 1-19 Superior Portion of the Spinal Cord . 23 Plate 1-20 Inferior Portion of the Spinal Cord . 24 Plate 1-21 Blood Supply of the Spinal Cord, Anterior View. 25 Plate 1-22 Venous Drainage of the Vertebral Column and Spinal Cord . 26 Plate 1-23 Dermatomes . 27 10752-01_CH01redo.qxd 9/24/07 3:07 PM Page 4 Palpable Structures of the Back PLATE 1-01 Palpable bony structures Superior nuchal line External occipital protuberance Superior border of trapezius muscle Vertebra prominens (C7) Clavicle Acromioclavicular Acromion of scapula joint Spine of scapula Greater tubercle Posterior axillary of humerus fold Inferior angle of scapula Spinous processes of vertebrae in vertebral furrow Rib Bulge of erector spinae muscles Iliac crest Hip bone Posterior superior iliac spine Sacrum Coccyx Greater trochanter of femur Ischial tuberosity Chapter 1 Lippincott Williams & Wilkins Atlas of Anatomy PAGE 5 10752-01_CH01redo.qxd 9/24/07 3:07 PM Page 6 PLATE 1-02 Vertebral Column, Lateral View Cervical Vertebrae PLATE 1-03 Vertebral Column, Lateral View A.