Aluminium - Wikipedia, the Free Encyclopedia Page 1 of 17
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Transport of Dangerous Goods
ST/SG/AC.10/1/Rev.16 (Vol.I) Recommendations on the TRANSPORT OF DANGEROUS GOODS Model Regulations Volume I Sixteenth revised edition UNITED NATIONS New York and Geneva, 2009 NOTE The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. ST/SG/AC.10/1/Rev.16 (Vol.I) Copyright © United Nations, 2009 All rights reserved. No part of this publication may, for sales purposes, be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, electrostatic, magnetic tape, mechanical, photocopying or otherwise, without prior permission in writing from the United Nations. UNITED NATIONS Sales No. E.09.VIII.2 ISBN 978-92-1-139136-7 (complete set of two volumes) ISSN 1014-5753 Volumes I and II not to be sold separately FOREWORD The Recommendations on the Transport of Dangerous Goods are addressed to governments and to the international organizations concerned with safety in the transport of dangerous goods. The first version, prepared by the United Nations Economic and Social Council's Committee of Experts on the Transport of Dangerous Goods, was published in 1956 (ST/ECA/43-E/CN.2/170). In response to developments in technology and the changing needs of users, they have been regularly amended and updated at succeeding sessions of the Committee of Experts pursuant to Resolution 645 G (XXIII) of 26 April 1957 of the Economic and Social Council and subsequent resolutions. -

Electromigration in Thin Films of Aluminium"
Co / Y A thesis entitled "ELECTROMIGRATION IN THIN FILMS OF ALUMINIUM" by Stephen Roberts B.Sc., A.R.C.S. Submitted for the Degree of Doctor of Philosophy of the University of London Imperial College of Science and Technology London 1982 2 ABSTRACT Electromigration is a common cause of failure of the aluminium thin film metallizations used in many microelectronic devices and circuits. The objective of the present work was to draw correlations between processing parameters, micro- structure and susceptibility to electromigration failure of vacuum-evaporated aluminium films on amorphous Si O^. The chemical composition of the Al - Si C>2 interface was examined using x-ray photoelectron spectroscopy, Auger electron spectroscopy and transmission electron microscopy and diffraction. The effects of substrate surface preparation and temperature on Al - Si O^ interfacial reaction were investigated. Reduction of the Si02 by the aluminium, resulting in the production of polycrystalline silicon and Tj - alumina, was found to occur under the conditions prevalent in many industrial metallization processes. The implications of Al-Si O2 reaction for device reliability are discussed. A range of techniques, including transmission and scanning electron microscopy and grazing incidence x-ray diffraction, were used to characterize the grain size, preferred orientation and surface topography of aluminium films deposited onto Si C>2 under various conditions. 1 yum thick aluminium films were found to have a ^111) fibre texture and the mean grain size ranged from less than 0.5yjm for deposition at 300 K, to ** 5 yum, for deposition at 675 K. Some thinner films were also examined, and trends in grain size and orientation with thickness were established. -

SAFETY DATA SHEET Revision Date 28.07.2021 According to Regulation (EC) No
Version 7.0 SAFETY DATA SHEET Revision Date 28.07.2021 according to Regulation (EC) No. 1907/2006 Print Date 02.10.2021 GENERIC EU MSDS - NO COUNTRY SPECIFIC DATA - NO OEL DATA SECTION 1: Identification of the substance/mixture and of the company/undertaking 1.1 Product identifiers Product name : Aluminum iodide Product Number : 208493 Brand : Aldrich REACH No. : A registration number is not available for this substance as the substance or its uses are exempted from registration, the annual tonnage does not require a registration or the registration is envisaged for a later registration deadline. CAS-No. : 7784-23-8 1.2 Relevant identified uses of the substance or mixture and uses advised against Identified uses : Laboratory chemicals, Manufacture of substances 1.3 Details of the supplier of the safety data sheet Company : Sigma-Aldrich Inc. 3050 SPRUCE ST ST. LOUIS MO 63103 UNITED STATES Telephone : +1 314 771-5765 Fax : +1 800 325-5052 1.4 Emergency telephone Emergency Phone # : 800-424-9300 CHEMTREC (USA) +1-703- 527-3887 CHEMTREC (International) 24 Hours/day; 7 Days/week SECTION 2: Hazards identification 2.1 Classification of the substance or mixture Classification according to Regulation (EC) No 1272/2008 Skin corrosion (Sub-category 1B), H314 Serious eye damage (Category 1), H318 For the full text of the H-Statements mentioned in this Section, see Section 16. 2.2 Label elements Labelling according Regulation (EC) No 1272/2008 Aldrich- 208493 Page 1 of 8 The life science business of Merck operates as MilliporeSigma in the US and Canada Pictogram Signal word Danger Hazard statement(s) H314 Causes severe skin burns and eye damage. -

Aryl‑NHC Group 13 Trimethyl Complexes : Structural, Stability and Bonding Insights
This document is downloaded from DR‑NTU (https://dr.ntu.edu.sg) Nanyang Technological University, Singapore. Aryl‑NHC group 13 trimethyl complexes : structural, stability and bonding insights Wu, Melissa Meiyi 2017 Wu, M. M. (2017). Aryl‑NHC group 13 trimethyl complexes : structural, stability and bonding insights. Doctoral thesis, Nanyang Technological University, Singapore. http://hdl.handle.net/10356/70204 https://doi.org/10.32657/10356/70204 Downloaded on 28 Sep 2021 13:14:06 SGT ATTENTION: The Singapore Copyright Act applies to the use of this document. Nanyang Technological University Library. NANYANG TECHNOLOGICAL UNIVERSITY DIVISION OF CHEMISTRY AND BIOLOGICAL CHEMISTRY SCHOOL OF PHYSICAL & MATHEMATICAL SCIENCES Aryl-NHC Group 13 Trimethyl Complexes: Structural, Stability and Bonding Insights Wu Meiyi Melissa G1102527F Supervisor: Asst Prof Felipe Garcia Contents Acknowledgements .............................................................................................................. iv Abbreviations ....................................................................................................................... v Abstract.............................................................................................................................. viii 1. Introduction 1.1. N-Heterocyclic Carbenes (NHC) ................................................................................. 1 1.1.1. Electronic Properties ............................................................................................ 1 1.1.2. Steric -

Hectorite: Synthesis, Modification, Assembly and Applications Jing Zhang, Chun Hui Zhou, Sabine Petit, Hao Zhang
Hectorite: Synthesis, modification, assembly and applications Jing Zhang, Chun Hui Zhou, Sabine Petit, Hao Zhang To cite this version: Jing Zhang, Chun Hui Zhou, Sabine Petit, Hao Zhang. Hectorite: Synthesis, modification, assembly and applications. Applied Clay Science, Elsevier, 2019, 177, pp.114-138. 10.1016/j.clay.2019.05.001. hal-02363196 HAL Id: hal-02363196 https://hal-cnrs.archives-ouvertes.fr/hal-02363196 Submitted on 2 Dec 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. 1 Hectorite:Synthesis, Modification, Assembly and Applications 2 3 Jing Zhanga, Chun Hui Zhoua,b,c*, Sabine Petitd, Hao Zhanga 4 5 a Research Group for Advanced Materials & Sustainable Catalysis (AMSC), State Key Laboratory 6 Breeding Base of Green Chemistry-Synthesis Technology, College of Chemical Engineering, Zhejiang 7 University of Technology, Hangzhou 310032, China 8 b Key Laboratory of Clay Minerals of Ministry of Land and Resources of the People's Republic of 9 China, Engineering Research Center of Non-metallic Minerals of Zhejiang Province, Zhejiang Institute 10 of Geology and Mineral Resource, Hangzhou 310007, China 11 c Qing Yang Institute for Industrial Minerals, You Hua, Qing Yang, Chi Zhou 242804, China 12 d Institut de Chimie des Milieux et Matériaux de Poitiers (IC2MP), UMR 7285 CNRS, Université de 13 Poitiers, Poitiers Cedex 9, France 14 15 Correspondence to: Prof. -
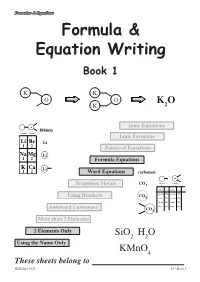
Formula & Equation Writing
Formulae & Equations Formula & Equation Writing Book 1 K K O O K O K 2 Ionic Equations lithium column column 1 2 Ionic Formulae Li Be Li 1 2 Balanced Equations Na Mg Li 1 2 Formula Equations K Ca Li 1 2 Word Equations carbonate valency valency Transition Metals CO3 1 2 name formula name formula ammonium NH4 carbonate CO3 Using Brackets CO 3 cyanide CN chromate CrO4 hydroxide OH sulphate SO4 nitrate NO sulphite SO Awkward Customers 3 3 CO3 More than 2 Elements 2 Elements Only SiO2 H2O Using the Name Only KMnO4 These sheets belong to KHS Sept 2013 page 1 S3 - Book 1 Formulae & Equations What is a Formula ? The formula of a compound tells you two things about the compound :- SiO H O i) which elements are in the compound 2 2 using symbols, and KMnO4 ii) how many atoms of each element are in the compound using numbers. Test Yourself What would be the formula for each of the following? Br H Cl K H C S Al Br O H Cl K H Br Using the Name Only Some compounds have extra information in their carbon monoxide names that allow people to work out and write CO the correct formula. carbon dioxide CO2 The names of the elements appear as usual but dinitrogen tetroxide N O this time the number of each type of atom is 2 4 included using mono- = 1 di- = 12 tri- = 3 tetra- = 4 penta- = 5 hexa- =46 Test Yourself 1 What would be the formula for each of the following? 1. -

Isomorphous Substitution of Aluminium for Silicon in Tobermoritic Structure
Isomorphous Substitution of Aluminium for Silicon in Tobermoritic Structure. I. The Mixtures of Different Forms of Silicon Dioxide and of Different Compounds of Aluminium J. PETROVIÖ, V. RUSNÁK and L. ŠTEVULA Institute of Inorganic Chemistry, Slovak Academy of Sciences, Bratislava 9 Received July 2, 1968 The isomorphous substitution of Al(III) for Si(IV) in tobermoritic structure was examined by X-ray phase analysis and DTA. It has been found that the extent of the substitution depends on the materials used. The samples were prepared using different forms of silica-either ^-quartz, quartz-glass, Si02-gel or aerosil. Gibbsite, boehmite, dehydrated kaolinite, kaolinite, corundum and 7-AI2O3 were used as sources of aluminium. The samples were heated in an autoclave at 150, 180 and 200°C for 10, 24 and in some cases for 48 hours. Tobermorite can be synthesized from calcium oxide and silica under hydrothermal conditions. Up to temperatures of about 110°C it is stable, at higher temperatures and under hydrothermal conditions it is formed as an unstable compound. When the reaction is allowed to proceed for a longer time, or when it takes place at higher tem perature, another stable calcium hydrosilicate arises. At lower temperature a calcium hydrosilicate with tobermoritic structure is formed. It has been found that tobermori te is produced on autoclaving building materials containing lime and some materials with high SÍO2 content. It is also formed from anhydrous dicalcium silicates in the course of setting of cement. Under different conditions, compounds with tobermoritic structure arise which, however, differ in the amount or lime and water in the molecu le, as well as in the arrangement of the structure — e.g. -

Wettability of Aluminium with Sic and Graphite in Aluminium Filtration
Light Metals 2011 Edited by: Stephen J. Lindsay TMS (The Minerals, Metals & Materials Society), 2011 WETTABILITY OF ALUMINIUM WITH SIC AND GRAPHITE IN ALUMINIUM FILTRATION Sarina Bao1, Anne Kvithyld2, Thorvald Abel Engh1, Merete Tangstad1 Norwegian University of Science and Technology, Trondheim, NO-7491, Norway 2SINTEF Materials and Chemistry, Trondheim, N-7465, Norway Keywords: Wettability, Contact angle, Aluminium, Graphite, SiC, Time dependent property Abstract éõõ The aim of aluminium filtration is to remove inclusions such as ] ■ SCSiC,Laurent,1996 . .nJ ! I · OxidizedSCSiC.Laurent ,1991 AI3C4. For inclusions to be removed they have to come in close '40j-■---;-- £-J.Ì A SSiC,Shimbo,1989 ] T ! : V SSiC,Han,1993 contact with the filter walls composed of A1203 or SiC. It is p I2O1 J.-Ä-..J. : + RBSiC,Han,1993 therefore important that the molten aluminium has close contact ^3 \ ! ! 6 SSiC,Candan,2002 OfHQfll ; ]..►. j I] • SCSiC,Laurent,1987 | with the filter wall. In addition to wetting properties between G J i inclusions (AI4C3) and molten aluminium, the wettability of the s eoi -i i *i i filter (SiC) by aluminium is determined in sessile drop studies in w the temperature range 1000-1300°C. Wettability changes with g 60I-- ! $ 4- time in three successive steps and improves with time. To 40 4 j -.<;- describe wettability at filtration temperatures employed in the Ö I ! industry of around 700°C, the results will be extrapolated to this 2èº ■—.—■—1—-—1—-—i ■—.—■—1—.—I temperature in future work. 600 700 800 900 1000 1100 1200 1300 Introduction Temperature/°C Figure 1 The equilibrium contact angle vs. -

Chemical Compatibility Between Aluminium Base Matrices and Light Refractory Carbide Reinforcements
CHEMICAL COMPATIBILITY BETWEEN ALUMINIUM BASE MATRICES AND LIGHT REFRACTORY CARBIDE REINFORCEMENTS J.C. Viala, M. Peronnet, F. Bosselet and J. Bouix Laboratoire des Multimatériaux et Interfaces, UMR CNRS 5615, Université Lyon 1, F 69622 Villeurbanne Cedex, France SUMMARY: The main features of the interface chemistry of Al/B4C, Al/TiC and Al/SiC couples at temperatures up to 1000 °C are presented and discussed in terms of thermodynamics, kinetics and reaction mechanism. The Al/B4C couple appears reactive whatever the temperature whereas a reactive-non reactive transition occurs at 812 °C on heating for the Al/TiC couple and at 650 °C on cooling for the Al/SiC couple. The reaction products are Al3BC and AlB2 or β-AlB12 for the Al/B4C couple, Al4C3 and Al3Ti for Al/TiC and finally Al4C3 and dissolved silicon for Al/SiC. Whatever the couple, interaction always proceeds via a dissolution-precipitation mechanism which ends in certain instances with the passivation of the base carbide surface by a continuous Al3BC or Al4C3 layer. Some examples are given illustrating how advantage can be taken of these features for interface tailoring. KEYWORDS: metal matrix, aluminium, boron carbide, titanium carbide, silicon carbide, interface, chemical reactions. INTRODUCTION The physical and mechanical characteristics of the light refractory carbides SiC, TiC and B4C make them suitable for being used as reinforcement in aluminium base metal matrix composites. Discontinuously reinforced composites or cermets have thus been produced by incorporating particles, small crystals or whiskers of these carbides in the metal matrix by various pyrometallurgic techniques [1-3]. Attempts have also been made to elaborate such products by in-situ reaction of carbon particles with appropriate alloy melts [4, 5]. -

Absence of Skin Sensitivity to Oxides of Aluminium, Silicon, Titanium Or Zirconium in Patients With
Gut1996;39:231-233 231 Absence of skin sensitivity to oxides of aluminium, Silicon, titanium or zirconium in patients with Crohn's disease Gut: first published as 10.1136/gut.39.2.231 on 1 August 1996. Downloaded from J C W Lee, S Halpem, D G Lowe, A Forbes, J E Lennard-Jones Abstract obstructive lymphadenopathy. It has been Background-Some metallic compounds, proposed that this is caused by fibrosis of the especially of zirconium, can cause cell afferent lymphatics as a result of absorption of mediated granulomatous inflammation of microparticles of silica and alumino-silicates the skin. Pigment granules containing through the skin where people walk barefoot compounds of aluminium, silicon, and on certain types of soil. Particles containing titanium have been observed within silica, titanium, and aluminium are present in macrophages in the wall of the small microgranulomata within inguinal lymph intestine in health and in Crohn's disease. nodes of sufferers.6 Granulomata also develop Zirconium compounds can be ingested in in response to intradermal injection ofcolloidal toothpaste. silica in healthy subjects but these are foreign Aim-To determine in a pilot study if body granulomata and are clearly distinguish- granulomatous sensitivity can be detected able from the cell mediated response to small to compounds of these metals or silicon quantities of zirconium lactate.7 after injection into the skin of patients As metals and minerals are ubiquitous in the with Crohn's disease. community, a hypersensitivity to these sub- Subjects-Eight patients with Crohn's stances in some people rather than a direct disease known to have had granulomata in toxic effect is the most probable pathogenetic the intestine and not currently treated mechanism by which they may contribute to with corticosteroids, and two healthy disease. -

Aluminium Carbide
safety data sheet according to Regulation (EC) No. 1907/2006 (REACH), amended by 2015/830/EU Aluminium carbide pure article number: 3094 date of compilation: 2017-01-17 Version: 1.0 en SECTION 1: Identification of the substance/mixture and of the company/undertaking 1.1 Product identifier Identification of the substance Aluminium carbide Article number 3094 Registration number (REACH) This information is not available. EC number 215-076-2 CAS number 1299-86-1 1.2 Relevant identified uses of the substance or mixture and uses advised against Identified uses: laboratory chemical 1.3 Details of the supplier of the safety data sheet Carl Roth GmbH + Co KG Schoemperlenstr. 3-5 D-76185 Karlsruhe Germany Telephone: +49 (0) 721 - 56 06 0 Telefax: +49 (0) 721 - 56 06 149 e-mail: [email protected] Website: www.carlroth.de Competent person responsible for the safety data : Department Health, Safety and Environment sheet e-mail (competent person) : [email protected] 1.4 Emergency telephone number Emergency information service Poison Centre Munich: +49/(0)89 19240 SECTION 2: Hazards identification 2.1 Classification of the substance or mixture Classification according to Regulation (EC) No 1272/2008 (CLP) Classification acc. to GHS Section Hazard class Hazard class and cat- Hazard egory state- ment 2.12 substance and mixture which, in contact with water, emits flam- (Water-react. 2) H261 mable gas 3.2 skin corrosion/irritation (Skin Irrit. 2) H315 3.3 serious eye damage/eye irritation (Eye Irrit. 2) H319 3.8R specific target organ toxicity - single exposure (respiratory tract ir- (STOT SE 3) H335 ritation) Ireland (en) Page 1 / 12 safety data sheet according to Regulation (EC) No. -

Developing Aluminum Nitride Nanoparticles: Chemical Synthesis
NanoPharma 2020 Der Pharmacia Sinica 2020 Vol.11 No.3 Developing Aluminum Nitride Nanoparticles: Chemical synthesis and Exploration of biocompatibility and anticancer activity against Cervical Cancer Cells Manjot Kaur 1, Kulwinder Singh 1, Paviter Singh 1, Amanpreet Kaur 1, 2, Ramovatar Meena 3, Gajendra 4 5 6 1 Pratap Singh , Hamed Barabadi , Muthupandian Saravanan *, Akshay Kumar 1Advanced Functional Material Lab., Department of Nanotechnology, Sri Guru Granth Sahib World University, Fatehgarh Sahib-140 407, Punjab, India 2Department of Physics, Sri Guru Granth Sahib World University, Fatehgarh Sahib-140 407, Punjab, India 3Nanotoxicology Laboratory, School of Environmental Sciences, Jawaharlal Nehru University, New Delhi-110067, India 4School of Computational and Integrated Sciences, Jawaharlal Nehru University, New Delhi-110 067, India 5Department of Pharmaceutical Biotechnology, School of Pharmacy, Shahid Beheshti University of Medical Sciences, Tehran, Iran 6Department of Medical Microbiology and Immunology, Division of Biomedical Sciences, School of Medicine, College of Health Sciences, Mekelle University, Mekelle, Ethiopia AlN nanoparticles were synthesized using a simple and sensors and implantable biomedical and effective solvothermal method. The X-ray diffraction results microelectromechanical devices. However, the development of revealed the cubic phase of AlN, and the field emission such materials is bound to one requirement, which is scanning electron microscopy analysis demonstrated the biocompatibility. The materials showing great chemical structural morphology of the synthesized materials. In addition, inertness, stability, and high compatibility with biological the cytotoxicity of the AlN nanoparticles was assessed against samples are considered optimal in this regard to be utilized in healthy (HEK-293, HUVEC, and MCF10A) and cancerous cell biomedical devices. The main benefit of the lower cytotoxicity line (HeLa).