Advertisement Call of Hyloxalus Elachyhistus (Edwards, 1971) (Anura, Dendrobatidae)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Advertisement Call of Pratt's Rocket Frog, Colostethus Pratti, from The
SALAMANDRA 56(4): 395–400 SALAMANDRA 30 October 2020 ISSN 0036–3375 Correspondence German Journal of Herpetology Correspondence Advertisement call of Pratt’s rocket frog, Colostethus pratti, from the western Andes of Colombia (Anura: Dendrobatidae) Juan David Jiménez-Bolaño1, Andrés Camilo Montes-Correa1, Franziska Leonhardt2 & Juan Manuel Renjifo1 1) Grupo de Investigación en Manejo & Conservación de Fauna, Flora & Ecosistemas Estratégicos Neotropicales (MIKU), Universidad del Magdalena, Santa Marta, Colombia 2) Museum of Zoology, Senckenberg Natural History Collections Dresden, A. B. Meyer Building, 01109 Dresden, Germany Corresponding author: Andrés Camilo Montes-Correa, e-mail: [email protected] Manuscript received: 22 July 2019 Accepted: 14 April 2020 by Stefan Lötters Northwestern South America is one of the biologically most phibian checklists (Cochran & Goin 1970, Ruiz-Car- diverse yet least known regions of the world. The Tropi- ranza et al. 1994, Acosta-Galvis 2000, Lynch & Suá- cal Andes and Chocó-Tumbes-Magdalena ecoregions, two rez-Mayorga 2004, Ballesteros-Correa et al. 2019). hotspots with high degrees of endemism and conservation The most recent information onC. pratti in Colombia con- priority (Myers et al. 2000, Rodríguez-Mahecha et al. cerns its altitudinal distribution, relative abundance, and 2004), are part of this region. Especially the Chocoan low- natural history in the northernmost part of the western lands and the western Andes of Colombia are areas with Andes, at the Serranía de San Jerónimo, Departamento de exceptional high amphibian diversity and many endemic Córdoba (Romero-Martínez et al. 2008, Ballesteros- taxa (e.g., Lynch et al. 1997, Lynch & Suárez-Mayorga Correa et al. 2019). To the best of our knowledge, there is 2004, Armesto & Señaris 2017). -

Chromosome Analysis of Five Brazilian
c Indian Academy of Sciences RESEARCH ARTICLE Chromosome analysis of five Brazilian species of poison frogs (Anura: Dendrobatidae) PAULA CAMARGO RODRIGUES1, ODAIR AGUIAR2, FLÁVIA SERPIERI1, ALBERTINA PIMENTEL LIMA3, MASAO UETANEBARO4 and SHIRLEI MARIA RECCO-PIMENTEL1∗ 1Departamento de Anatomia, Biologia Celular e Fisiologia, Instituto de Biologia, Universidade Estadual de Campinas, 13083-863 Campinas, São Paulo, Brazil 2Departamento de Biociências, Universidade Federal de São Paulo, Campus Baixada Santista, 11060-001 Santos, São Paulo, Brazil 3Coordenadoria de Pesquisas em Ecologia, Instituto Nacional de Pesquisas do Amazonas, 69011-970 Manaus, Amazonas, Brazil 4Departamento de Biologia, Universidade Federal de Mato Grosso do Sul, 70070-900 Campo Grande, Mato Grosso do Sul, Brazil Abstract Dendrobatid frogs have undergone an extensive systematic reorganization based on recent molecular findings. The present work describes karyotypes of the Brazilian species Adelphobates castaneoticus, A. quinquevittatus, Ameerega picta, A. galactonotus and Dendrobates tinctorius which were compared to each other and with previously described related species. All karyotypes consisted of 2n = 18 chromosomes, except for A. picta which had 2n = 24. The karyotypes of the Adelphobates and D. tinctorius species were highly similar to each other and to the other 2n = 18 previously studied species, revealing conserved karyotypic characteristics in both genera. In recent phylogenetic studies, all Adelphobates species were grouped in a clade separated from the Dendrobates species. Thus, we hypothesized that their common karyotypic traits may have a distinct origin by chromosome rearrangements and mutations. In A. picta, with 2n = 24, chromosome features of pairs from 1 to 8 are shared with other previously karyotyped species within this genus. Hence, the A. -
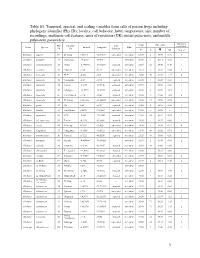
1 Table S1. Temporal, Spectral, and Scaling Variables from Calls Of
Table S1. Temporal, spectral, and scaling variables from calls of poison frogs including phylogeny identifier (Phy ID), locality, call behavior, habit, temperature, size, number of recordings, multinote call features, units of repetition (UR), initial pulse-note, and middle pulse-note parameters. Analyzed Phy Locality Call Temp SVL (mm) Genus Species Latitude Longitude Habit recordings ID ID Behavior °C N ! SD N of ♂ Allobates algorei 60 El Tama 7.65375 -72.19137 concealed terrestrial 23.50 8 18.90 0.70 3 Allobates brunneus 37 Guimaraes -15.2667 -55.5311 -- terrestrial 26.50 1 16.13 0.00 1 Allobates caeruleodactylus 48 Borba -4.398593 -59.60251 exposed terrestrial 25.60 12 15.50 0.40 1 Allobates crombiei 52 Altamira -3.65 -52.38 concealed terrestrial 24.10 2 18.10 0.04 2 Allobates femoralis 43 ECY -0.633 -76.5 concealed terrestrial 25.60 20 23.58 1.27 6 Allobates femoralis 46 Porongaba -8.67 -72.78 exposed terrestrial 25.00 1 25.38 0.00 1 Allobates femoralis 44 Leticia -4.2153 -69.9406 exposed terrestrial 25.50 1 20.90 0.00 1 Allobates femoralis 40 Albergue -12.8773 -71.3865 exposed terrestrial 26.00 6 21.98 2.18 1 Allobates femoralis 41 CAmazonico -12.6 -70.08 exposed terrestrial 26.00 12 22.43 1.06 4 Allobates femoralis 45 El Palmar 8.333333 -61.66667 concealed terrestrial 24.00 27 25.50 0.76 1 Allobates granti 49 FG 3.62 -53.17 exposed terrestrial 24.60 8 16.15 0.55 1 Allobates humilis 59 San Ramon 8.8678 -70.4861 concealed terrestrial 19.50 -- 21.80 -- 1 Allobates insperatus 54 ECY -0.633 -76.4005 exposed terrestrial 24.60 18 16.64 0.93 7 Allobates aff. -

Anura: Dendrobatidae: Anomaloglossus) from the Orinoquian Rainforest, Southern Venezuela
TERMS OF USE This pdf is provided by Magnolia Press for private/research use. Commercial sale or deposition in a public library or website is prohibited. Zootaxa 2413: 37–50 (2010) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Article ZOOTAXA Copyright © 2010 · Magnolia Press ISSN 1175-5334 (online edition) A new dendrobatid frog (Anura: Dendrobatidae: Anomaloglossus) from the Orinoquian rainforest, southern Venezuela CÉSAR L. BARRIO-AMORÓS1,4, JUAN CARLOS SANTOS2 & OLGA JOVANOVIC3 1,4 Fundación AndígenA, Apartado postal 210, 5101-A Mérida, Venezuela 2University of Texas at Austin, Integrative Biology, 1 University Station C0930 Austin TX 78705, USA 3Division of Evolutionary Biology, Technical University of Braunschweig, Spielmannstr 8, 38106 Braunschweig, Germany 4Corresponding author. E-mail: [email protected]; [email protected] Abstract A new species of Anomaloglossus is described from the Venezuelan Guayana; it is the 21st described species of Anomaloglossus from the Guiana Shield, and the 15th from Venezuela. This species inhabits rainforest on granitic substrate on the northwestern edge of the Guiana Shield (Estado Amazonas, Venezuela). The new species is distinguished from congeners by sexual dimorphism, its unique male color pattern (including two bright orange parotoid marks and two orange paracloacal spots on dark brown background), call characteristics and other morphological features. Though to the new species is known only from the northwestern edge of the Guiana Shield, its distribution may be more extensive, as there are no significant biogeographic barriers isolating the type locality from the granitic lowlands of Venezuela. Key words: Amphibia, Dendrobatidae, Anomaloglossus, Venezuela, Guiana Shield Resumen Se describe una nueva especie de Anomaloglossus de la Guayana venezolana, que es la vigesimoprimera descrita del Escudo Guayanés, y la decimoquinta para Venezuela. -

A Review of Chemical Defense in Poison Frogs (Dendrobatidae): Ecology, Pharmacokinetics, and Autoresistance
Chapter 21 A Review of Chemical Defense in Poison Frogs (Dendrobatidae): Ecology, Pharmacokinetics, and Autoresistance Juan C. Santos , Rebecca D. Tarvin , and Lauren A. O’Connell 21.1 Introduction Chemical defense has evolved multiple times in nearly every major group of life, from snakes and insects to bacteria and plants (Mebs 2002 ). However, among land vertebrates, chemical defenses are restricted to a few monophyletic groups (i.e., clades). Most of these are amphibians and snakes, but a few rare origins (e.g., Pitohui birds) have stimulated research on acquired chemical defenses (Dumbacher et al. 1992 ). Selective pressures that lead to defense are usually associated with an organ- ism’s limited ability to escape predation or conspicuous behaviors and phenotypes that increase detectability by predators (e.g., diurnality or mating calls) (Speed and Ruxton 2005 ). Defended organisms frequently evolve warning signals to advertise their defense, a phenomenon known as aposematism (Mappes et al. 2005 ). Warning signals such as conspicuous coloration unambiguously inform predators that there will be a substantial cost if they proceed with attack or consumption of the defended prey (Mappes et al. 2005 ). However, aposematism is likely more complex than the simple pairing of signal and defense, encompassing a series of traits (i.e., the apose- matic syndrome) that alter morphology, physiology, and behavior (Mappes and J. C. Santos (*) Department of Zoology, Biodiversity Research Centre , University of British Columbia , #4200-6270 University Blvd , Vancouver , BC , Canada , V6T 1Z4 e-mail: [email protected] R. D. Tarvin University of Texas at Austin , 2415 Speedway Stop C0990 , Austin , TX 78712 , USA e-mail: [email protected] L. -

Taxonomic Checklist of Amphibian Species Listed in the CITES
CoP17 Doc. 81.1 Annex 5 (English only / Únicamente en inglés / Seulement en anglais) Taxonomic Checklist of Amphibian Species listed in the CITES Appendices and the Annexes of EC Regulation 338/97 Species information extracted from FROST, D. R. (2015) "Amphibian Species of the World, an online Reference" V. 6.0 (as of May 2015) Copyright © 1998-2015, Darrel Frost and TheAmericanMuseum of Natural History. All Rights Reserved. Additional comments included by the Nomenclature Specialist of the CITES Animals Committee (indicated by "NC comment") Reproduction for commercial purposes prohibited. CoP17 Doc. 81.1 Annex 5 - p. 1 Amphibian Species covered by this Checklist listed by listed by CITES EC- as well as Family Species Regulation EC 338/97 Regulation only 338/97 ANURA Aromobatidae Allobates femoralis X Aromobatidae Allobates hodli X Aromobatidae Allobates myersi X Aromobatidae Allobates zaparo X Aromobatidae Anomaloglossus rufulus X Bufonidae Altiphrynoides malcolmi X Bufonidae Altiphrynoides osgoodi X Bufonidae Amietophrynus channingi X Bufonidae Amietophrynus superciliaris X Bufonidae Atelopus zeteki X Bufonidae Incilius periglenes X Bufonidae Nectophrynoides asperginis X Bufonidae Nectophrynoides cryptus X Bufonidae Nectophrynoides frontierei X Bufonidae Nectophrynoides laevis X Bufonidae Nectophrynoides laticeps X Bufonidae Nectophrynoides minutus X Bufonidae Nectophrynoides paulae X Bufonidae Nectophrynoides poyntoni X Bufonidae Nectophrynoides pseudotornieri X Bufonidae Nectophrynoides tornieri X Bufonidae Nectophrynoides vestergaardi -

Reproductive Biology of Ameerega Trivittata(Anura: Dendrobatidae)
ACTA AMAZONICA http://dx.doi.org/10.1590/1809-4392201305384 Reproductive biology of Ameerega trivittata (Anura: Dendrobatidae) in an area of terra firme forest in eastern Amazonia Ellen Cristina Serrão ACIOLI1 * , Selvino NECKEL-OLIVEIRA2 ¹ Universidade Federal do Pará/Museu Paraense Emílio Goeldi, Programa de Pós Graduação em Zoologia, Av. Perimetral 1901/1907, Terra Firme, 66017-970, Belém, Pará, Brasil. ² Universidade Federal de Santa Catarina, Departamento de Ecologia e Zoologia, Centro de Ciências Biológicas, 88040-970, Florianópolis, Santa Catarina, Brasil. * Corresponding author: [email protected] ABSTRACT The reproductive success of tropical amphibians is influenced by factors such as body size and the characteristics of breeding sites. Data on reproductive biology are important for the understanding of population dynamics and the maintenance of species. The objectives of the present study were to examine the abundance ofAmeerega trivittata, analyze the use of microhabitats by calling males and the snout-vent length (SVL) of breeding males and females, the number of tadpoles carried by the males and mature oocytes in the females, as well as the relationship between the SVL of the female and both the number and mean size of the mature oocytes found in the ovaries. Three field trips were conducted between January and September, 2009. A total of 31 plots, with a mean area of 2.3 ha, were surveyed, resulting in records of 235 individuals, with a mean density of 3.26 individuals per hectare. Overall, 66.1% of the individuals sighted were located in the leaf litter, while 17.4% were perched on decaying tree trunks on the forest floor, 15.7% on the aerial roots ofCecropia trees, and 0.8% on lianas. -

Summary Record of the 26Th Meeting of the Animals Committee
Original language: English AC26 summary record CONVENTION ON INTERNATIONAL TRADE IN ENDANGERED SPECIES OF WILD FAUNA AND FLORA ____________ Twenty-sixth meeting of the Animals Committee Geneva (Switzerland), 15-20 March 2012 and Dublin (Ireland), 22-24 March 2012 SUMMARY RECORD Animals Committee matters 1. Opening of the meeting The Chair opened the meeting and welcomed all participants, before giving the floor to the Secretary- General, who also welcomed everyone and introduced new members of the Secretariat's scientific team (Mr De Meulenaer and Ms Kwitsinskaia) and enforcement team (Ms Garcia Ferreira, Ms Jonsson and Mr van Rensburg). He wished the Committee well in its deliberations. The Chair thanked the Secretary-General and invited suggestions as to how the Conference of the Parties could establish stronger measures to support the Committee as well as export countries, which deserved particular assistance. No other intervention was made during discussion of this item.1 2. Rules of Procedure The Secretariat introduced document AC26 Doc. 2 and proposed amending Rule 22 as follows: “On request, the Secretariat shall distribute printed and translated documents...”. The Secretariat explained that most members regularly indicated that they did not need printed copies and that this proposal was made to reduce costs. Although not opposed to the change in principle, a Party regretted that the suggestion had not been presented in the document, which would have given Parties time to consider it, and was concerned that this unannounced proposal might create a precedent. Another Party asked a question on the procedure to accept observers, but the Chair invited it to raise this topic under agenda item 4 on Admission of observers. -

Two New Endangered Species of Anomaloglossus (Anura: Aromobatidae) from Roraima State, Northern Brazil
Zootaxa 3926 (2): 191–210 ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Article ZOOTAXA Copyright © 2015 Magnolia Press ISSN 1175-5334 (online edition) http://dx.doi.org/10.11646/zootaxa.3926.2.2 http://zoobank.org/urn:lsid:zoobank.org:pub:BCA3901A-DF07-4FAF-8386-C24649557313 Two new endangered species of Anomaloglossus (Anura: Aromobatidae) from Roraima State, northern Brazil ANTOINE FOUQUET1,2,8, SERGIO MARQUES SOUZA2, PEDRO M. SALES NUNES2,3, PHILIPPE J. R. KOK4,5, FELIPE FRANCO CURCIO2,6, CELSO MORATO DE CARVALHO7, TARAN GRANT2 & MIGUEL TREFAUT RODRIGUES2 1CNRS Guyane USR3456, Immeuble Le Relais, 2 Avenue Gustave Charlery, 97300, Cayenne, French Guiana 2Universidade de São Paulo, Instituto de Biociências, Departamento de Zoologia, Caixa Postal 11.461,CEP 05508-090, São Paulo, SP, Brazil 3Universidade Federal de Pernambuco, Centro de Ciências Biológicas, Departamento de Zoologia, Av. Professor Moraes Rego, s/n. Cidade Universitária CEP 50670-901, Recife, PE, Brazil 4Biology Department, Amphibian Evolution Lab, Vrije Universiteit Brussel, 2 Pleinlaan, B- 1050 Brussels, Belgium 5Department of Recent Vertebrates, Royal Belgian Institute of Natural Sciences, 29 rue Vautier, B- 1000 Brussels, Belgium 6Universidade Federal de Mato Grosso, Instituto de Biociências, Departamento de Biologia e Zoologia, CEP 78060-900, Cuiaba MT, Brazil 7INPA Núcleo de Pesquisas de Roraima (INPA/NPRR), Rua Coronel Pinto 315 – Centro, 69301-970, Boa Vista, RR, Brazil 8Corresponding author. E-mail: [email protected] Abstract We describe two new species of Anomaloglossus from Roraima State, Brazil, that are likely endemic to single mountains currently isolated among lowland forest and savanna ecosystems. The first species, Anomaloglossus tepequem sp. -

Myers 1987:304. Dendrobates Bombetes: Jungfer, Lötters, And
1 AMPHIBIA: ANURA: DENDROBATIDAE Andinobates bombetes Catalogue of American Amphibians and Minyobates bombetes: Myers 1987:304. Reptiles 926 Dendrobates bombetes: Jungfer, Lötters, and Jörgens 2000:11. F. Vargas-Salinas, M. A. Atehortua-Vallejo, L. Ranitomeya bombetes: Grant, Frost, Caldwell, F. Arcila-Pérez, G. M. Jiménez-Vargas, Gagliardo, Haddad, Kok, Means, Noonan, C. González-Acosta, S. Casas-Cardona, Schargel, and Wheeler 2006:171. and A. Grajales-Echeverry. 2020. Andinobates bombetes: Brown, Twomey, Andinobates bombetes. Amézquita, de Souza, Caldwell, Lötters, May, Melo-Sampaio, Mejía-Vargas, Pe- Andinobates bombetes (Myers and Daly) rez-Peña, Pepper, Poelman, Sanchez-Ro- Rubí poison frog driguez, and Summers 2011:36. Dendrobates bombetes Myers and Daly CONTENT. No subspecies are recognized. 1980:2. Type locality: “mountains above south side of Lago de Calima, 1580–1600 DESCRIPTION. Individuals of Andinobates meters elevation, about 2 km.,{sic} airline bombetes have a body size (snout-vent length, southwest of Puente Tierra (village), De- SVL) between 16.7– 21.5 mm, with no sexual partment of Valle del Cauca, Colombia.” dimorphism in body size (males: mean SVL Holotype, American Museum of Natural = 17.8 ± 0.1 mm SD, range: 16.7–21.5 mm; History, AMNH 102601, adult female, N = 28; females: mean SVL = 18.6 ± 0.1 mm collected by C. W. Myers, J. W. Daly and SD, range: 17.2–19.8 mm; N = 19) (Myers and E. B. de Bernal on 21 November 1976 (not Daly 1980; Suárez-Mayorga 1999; Vargas-Sa- examined by authors). linas and Amézquita 2013). In adults of Andi- Figure 1. Male of Andinobates bombetes from Finca El Placer, in the municipality of Filandia, depart- ment of Quindío, Colombia. -

Systematics of the Hyloxalus Bocagei Complex (Anura: Dendrobatidae), Description of Two New Cryptic Species, and Recognition of H
Zootaxa 2711: 1–75 (2010) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Monograph ZOOTAXA Copyright © 2010 · Magnolia Press ISSN 1175-5334 (online edition) ZOOTAXA 2711 Systematics of the Hyloxalus bocagei complex (Anura: Dendrobatidae), description of two new cryptic species, and recognition of H. maculosus MÓNICA I. PÁEZ-VACAS1,3, LUIS A. COLOMA1 & JUAN C. SANTOS2,4 1Museo de Zoología, Escuela de Biología, Pontificia Universidad Católica del Ecuador, Av. 12 de Octubre 1076 y Roca, Aptdo. 17-01-2184, Quito, Ecuador. E-mail: [email protected], [email protected] 2Section of Integrative Biology C0930, The University of Texas, Austin, TX 78712, USA 3Current address: Department of Biology, Colorado State University, Fort Collins, CO 80523-1878, USA 4Current address: National Evolutionary Synthesis Center, 2024 W, Main Street, Suite A200, Durham, NC 27705-4667, USA E-mail: [email protected] Magnolia Press Auckland, New Zealand Accepted by M. Vences: 28 Sep. 2010; published: 3 Dec. 2010 MÓNICA I. PÁEZ-VACAS, LUIS A. COLOMA & JUAN C. SANTOS Systematics of the Hyloxalus bocagei complex (Anura: Dendrobatidae), description of two new cryptic species, and recognition of H. maculosus (Zootaxa 2711) 75 pp.; 30 cm. 3 Dec. 2010 ISBN 978-1-86977-641-1 (paperback) ISBN 978-1-86977-642-8 (Online edition) FIRST PUBLISHED IN 2010 BY Magnolia Press P.O. Box 41-383 Auckland 1346 New Zealand e-mail: [email protected] http://www.mapress.com/zootaxa/ © 2010 Magnolia Press All rights reserved. No part of this publication may be reproduced, stored, transmitted or disseminated, in any form, or by any means, without prior written permission from the publisher, to whom all requests to reproduce copyright material should be directed in writing. -

Differences in the Diversity of Frogspecies Between Sierra Lloronaand El Valle, Panama Kei Okabe Thurber SIT Study Abroad
SIT Graduate Institute/SIT Study Abroad SIT Digital Collections Independent Study Project (ISP) Collection SIT Study Abroad Fall 12-6-2014 Differences in the Diversity of FrogSpecies between Sierra Lloronaand El Valle, Panama Kei Okabe Thurber SIT Study Abroad Follow this and additional works at: https://digitalcollections.sit.edu/isp_collection Part of the Biodiversity Commons, Environmental Health and Protection Commons, Environmental Indicators and Impact Assessment Commons, Latin American Studies Commons, Other Animal Sciences Commons, Population Biology Commons, and the Terrestrial and Aquatic Ecology Commons Recommended Citation Thurber, Kei Okabe, "Differences in the Diversity of FrogSpecies between Sierra Lloronaand El Valle, Panama" (2014). Independent Study Project (ISP) Collection. 2000. https://digitalcollections.sit.edu/isp_collection/2000 This Article is brought to you for free and open access by the SIT Study Abroad at SIT Digital Collections. It has been accepted for inclusion in Independent Study Project (ISP) Collection by an authorized administrator of SIT Digital Collections. For more information, please contact [email protected]. Differences in the Diversity of Frog Species between Sierra Llorona and El Valle, Panama By Kei Thurber SIT Panama, Fall 2014 Thurber 2 Table of Contents Acknowledgements ................................................................. 3 Abstract ................................................................................... 4 Resumen ................................................................................