Chemistry Level 4C (Chm415109)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Material Safety Data Sheet
LTS Research Laboratories, Inc. Safety Data Sheet Calcium Selenide ––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––– 1. Product and Company Identification ––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––– Trade Name: Calcium Selenide Chemical Formula: CaSe Recommended Use: Scientific research and development Manufacturer/Supplier: LTS Research Laboratories, Inc. Street: 37 Ramland Road City: Orangeburg State: New York Zip Code: 10962 Country: USA Tel #: 855-587-2436 / 855-lts-chem 24-Hour Emergency Contact: 800-424-9300 (US & Canada) +1-703-527-3887 (International) ––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––– 2. Hazards Identification ––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––– Signal Word: Danger Hazard Statements: H301+H331: Toxic if swallowed or if inhaled H373: May cause damage to organs through prolonged or repeated exposure H400: Very toxic to aquatic life H410: Very toxic to aquatic life with long lasting effects Precautionary Statements: P260: Do not breathe dust/fume/gas/mist/vapours/spray P261: Avoid breathing dust/fume/gas/mist/vapours/spray P264: Wash thoroughly after handling P270: Do not eat, drink or smoke when using this product P271: Use only outdoors or in a well-ventilated area P273: Avoid release to the environment P301+P310: IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician P304+P341: IF INHALED: If breathing is difficult, -

High Purity Inorganics
High Purity Inorganics www.alfa.com INCLUDING: • Puratronic® High Purity Inorganics • Ultra Dry Anhydrous Materials • REacton® Rare Earth Products www.alfa.com Where Science Meets Service High Purity Inorganics from Alfa Aesar Known worldwide as a leading manufacturer of high purity inorganic compounds, Alfa Aesar produces thousands of distinct materials to exacting standards for research, development and production applications. Custom production and packaging services are part of our regular offering. Our brands are recognized for purity and quality and are backed up by technical and sales teams dedicated to providing the best service. This catalog contains only a selection of our wide range of high purity inorganic materials. Many more products from our full range of over 46,000 items are available in our main catalog or online at www.alfa.com. APPLICATION FOR INORGANICS High Purity Products for Crystal Growth Typically, materials are manufactured to 99.995+% purity levels (metals basis). All materials are manufactured to have suitably low chloride, nitrate, sulfate and water content. Products include: • Lutetium(III) oxide • Niobium(V) oxide • Potassium carbonate • Sodium fluoride • Thulium(III) oxide • Tungsten(VI) oxide About Us GLOBAL INVENTORY The majority of our high purity inorganic compounds and related products are available in research and development quantities from stock. We also supply most products from stock in semi-bulk or bulk quantities. Many are in regular production and are available in bulk for next day shipment. Our experience in manufacturing, sourcing and handling a wide range of products enables us to respond quickly and efficiently to your needs. CUSTOM SYNTHESIS We offer flexible custom manufacturing services with the assurance of quality and confidentiality. -

Chemical Names and CAS Numbers Final
Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number C3H8O 1‐propanol C4H7BrO2 2‐bromobutyric acid 80‐58‐0 GeH3COOH 2‐germaacetic acid C4H10 2‐methylpropane 75‐28‐5 C3H8O 2‐propanol 67‐63‐0 C6H10O3 4‐acetylbutyric acid 448671 C4H7BrO2 4‐bromobutyric acid 2623‐87‐2 CH3CHO acetaldehyde CH3CONH2 acetamide C8H9NO2 acetaminophen 103‐90‐2 − C2H3O2 acetate ion − CH3COO acetate ion C2H4O2 acetic acid 64‐19‐7 CH3COOH acetic acid (CH3)2CO acetone CH3COCl acetyl chloride C2H2 acetylene 74‐86‐2 HCCH acetylene C9H8O4 acetylsalicylic acid 50‐78‐2 H2C(CH)CN acrylonitrile C3H7NO2 Ala C3H7NO2 alanine 56‐41‐7 NaAlSi3O3 albite AlSb aluminium antimonide 25152‐52‐7 AlAs aluminium arsenide 22831‐42‐1 AlBO2 aluminium borate 61279‐70‐7 AlBO aluminium boron oxide 12041‐48‐4 AlBr3 aluminium bromide 7727‐15‐3 AlBr3•6H2O aluminium bromide hexahydrate 2149397 AlCl4Cs aluminium caesium tetrachloride 17992‐03‐9 AlCl3 aluminium chloride (anhydrous) 7446‐70‐0 AlCl3•6H2O aluminium chloride hexahydrate 7784‐13‐6 AlClO aluminium chloride oxide 13596‐11‐7 AlB2 aluminium diboride 12041‐50‐8 AlF2 aluminium difluoride 13569‐23‐8 AlF2O aluminium difluoride oxide 38344‐66‐0 AlB12 aluminium dodecaboride 12041‐54‐2 Al2F6 aluminium fluoride 17949‐86‐9 AlF3 aluminium fluoride 7784‐18‐1 Al(CHO2)3 aluminium formate 7360‐53‐4 1 of 75 Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number Al(OH)3 aluminium hydroxide 21645‐51‐2 Al2I6 aluminium iodide 18898‐35‐6 AlI3 aluminium iodide 7784‐23‐8 AlBr aluminium monobromide 22359‐97‐3 AlCl aluminium monochloride -

Mass Balance and Trapping of Headspace Samples Of
MASS BALANCE AND TRAPPING OF HEADSPACE SAMPLES OF BIOREMEDIATION IN SELENIUM AMENDED SAMPLES ________________________________ A THESIS PRESENTED TO THE FACULTY OF THE DEPARTMENT OF CHEMISTRY SAM HOUSTON STATE UNIVERSITY ________________________________ IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF SCIENCE ________________________________ BY SUMINDA HAPUARACHCHI AUGUST, 2002 2 MASS BALANCE AND TRAPPING OF HEADSPACE SAMPLES OF BIOREMEDIATION IN SELENIUM AMENDED SAMPLES BY SUMINDA HAPUARACHCHI APPROVED: ________________________________ THOMAS G. CHASTEEN, THESIS DIRECTOR ________________________________ BENNY E. ARNEY JR. ________________________________ Mike McCann APPROVED: ________________________________ Dr. Brian Chapman, Dean College of Arts and Sciences 3 ABSTRACT Hapuarachchi, Suminda, Mass balance and trapping of headspace samples of bioremediation in selenium amended samples, Master of Science (Chemistry), August 2002, Sam Houston State University, Huntsville, Texas. One of the purposes of these experiments was to determine the mass balance in the bioremediation of selenium amended samples and to try to improve the efficiency of the bioremediation of selenium. The other purpose was to study the interference of glass containers in selenium determination by atomic absorption spectrometry with hydride generation. In this research, the first step was to develop a gas phase trapping method and to prove the success of that method using known concentrations of known organo-selenium compounds. Fifty percent nitric solution was a good trapping solution to collect volatile organoselenium compounds purged from live, liquid bacterial cultures. Then, bioreactor experiments were carried out to determine the mass balance of selenium as it was biologically processed by a selenium-resistant bacterium. First, bioreactor experiments were undertaken anaerobically with different amended selenium concentrations and the mass balance of each process measured. -

The Farewell Demos Andy Cherkas Stouffville DSS Retired [email protected]
The Farewell Demos Andy Cherkas Stouffville DSS Retired [email protected] Edit any lab or demonstration you may wish to do for your purposes. For example take out answers so the students can think about them. Try each before having students do them or before you demonstrate them to make sure everything will go as expected. Also remember that any demonstration can be reworked into a hands on lab for students and any lab for students can be demonstrated by the teacher. Any questions about these labs and demos should be directed towards myself or another experienced chemistry teacher that you have contacts with. Demo or Lab: CORROSION LAB Problem: What are the factors that slow and speed corrosion? Materials: eight clean test tubes, distilled water, tap water, common nails cleaned to remove any oil coating, painted nails, galvanized nails, copper wire, salt, acid, magnesium ribbon, 0.10 mol/L potassium ferricyanide solution Safety: Follow general safety procedures. Procedure: Prelab all the set up so students can come in the next day and start the set up. 1. Place a common nail in tap water in a test tube, with the nail head just out of the water 2. Place a 2nd nail in a second test tube completely covered by the tap water 3. Place a 3rd nail in distilled water in a test tube, with the nail head just out of the water 4. Place a 4th nail in the test tube, completely in tap water with salt added 5. Place a 5th nail in the test tube, completely in tap water with 5 drops of acid [vinegar will do] 6. -

HYSYS OLI Interface
HYSYS® 2004.2 OLI Interface Reference Guide Copyright October 2005 Copyright © 1981-2005 by Aspen Technology, Inc. All rights reserved. Aspen Accounting.21™, Aspen ACM Model Export, Aspen ACOL™, Aspen ACX™ Upgrade to ACOL™, Aspen Adsim®, Aspen Advisor™, Aspen Aerotran®, Aspen Alarm & Event™, Aspen APLE™, Aspen Apollo™, Aspen AtOMS™, Aspen Batch and Event Extractor, Aspen Batch Plus®, Aspen Batch.21™, Aspen Batch.21™ CBT, Aspen BatchCAD™, Aspen BatchSep™, Aspen Blend Model Library™, Aspen Blend™, Aspen BP Crude Oil Database, Aspen Calc CBT, Aspen Calc™, Aspen Capable-to-Promise®, Aspen CatRef®, Aspen Chromatography®, Aspen Cim-IO Core™, Aspen Cim-IO™ for @AGlance, Aspen Cim-IO™ for ABB 1180/ 1190 via DIU, Aspen Cim-IO™ for Bailey SemAPI, Aspen Cim-IO™ for DDE, Aspen Cim-IO™ for Eurotherm Gauge via DCP, Aspen Cim-IO™ for Fisher-Rosemount Chip, Aspen Cim-IO™ for Fisher-Rosemount RNI, Aspen Cim-IO™ for Foxboro FOXAPI, Aspen Cim-IO™ for G2, Aspen Cim-IO™ for GE FANUC via HCT, Aspen Cim-IO™ for Hitachi Ex Series, Aspen Cim-IO™ for Honeywell TDC 3000 via HTL/access, Aspen Cim-IO™ for Intellution Fix, Aspen Cim-IO™ for Measurex MCN, Aspen Cim-IO™ for Measurex ODX, Aspen Cim-IO™ for Moore Apacs via Nim (RNI), Aspen Cim-IO™ for OPC, Aspen Cim-IO™ for PI, Aspen Cim- IO™ for RSLinx, Aspen Cim-IO™ for SetCim/InfoPlus-X/InfoPlus.21, Aspen Cim-IO™ for Toshiba Tosdic, Aspen Cim-IO™ for ULMA 3D, Aspen Cim-IO™ for Westinghouse, Aspen Cim-IO™ for WonderWare InTouch, Aspen Cim-IO™ for Yokogawa ACG10S, Aspen Cim-IO™ for Yokogawa EW3, Aspen Collaborative Forecasting™, -
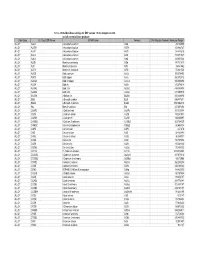
Database Full Listing
16-Nov-06 OLI Data Base Listings for ESP version 7.0.46, Analyzers 2.0.46 and all current alliance products Data Base OLI Tag (ESP) Name IUPAC Name Formula CAS Registry Number Molecular Weight ALLOY AL2U 2-Aluminum uranium Al2U 291.98999 ALLOY AL3TH 3-Aluminum thorium Al3Th 312.982727 ALLOY AL3TI 3-Aluminum titanium Al3Ti 128.824615 ALLOY AL3U 3-Aluminum uranium Al3U 318.971527 ALLOY AL4U 4-Aluminum uranium Al4U 345.953064 ALLOY ALSB Aluminum antimony AlSb 148.731537 ALLOY ALTI Aluminum titanium AlTi 74.861542 ALLOY ALTI3 Aluminum 3-titanium AlTi3 170.621536 ALLOY AUCD Gold cadmium AuCd 309.376495 ALLOY AUCU Gold copper AuCu 260.512512 ALLOY AUCU3 Gold 3-copper AuCu3 387.604492 ALLOY AUSN Gold tin AuSn 315.676514 ALLOY AUSN2 Gold 2-tin AuSn2 434.386505 ALLOY AUSN4 Gold 4-tin AuSn4 671.806519 ALLOY BA2SN 2-Barium tin Ba2Sn 393.369995 ALLOY BI2U 2-Bismuth uranium Bi2U 655.987671 ALLOY BI4U3 4-Bismuth 3-uranium Bi4U3 1550.002319 ALLOY BIU Bismuth uranium BiU 447.007294 ALLOY CA2PB 2-Calcium lead Ca2Pb 287.355988 ALLOY CA2SI 2-Calcium silicon Ca2Si 108.241501 ALLOY CA2SN 2-Calcium tin Ca2Sn 198.865997 ALLOY CA3SB2 3-Calcium 2-antimony Ca3Sb2 363.734009 ALLOY CAMG2 Calcium 2-magnesium CaMg2 88.688004 ALLOY CAPB Calcium lead CaPb 247.278 ALLOY CASI Calcium silicon CaSi 68.163498 ALLOY CASI2 Calcium 2-silicon CaSi2 96.249001 ALLOY CASN Calcium tin CaSn 158.787994 ALLOY CAZN Calcium zinc CaZn 105.468002 ALLOY CAZN2 Calcium 2-zinc CaZn2 170.858002 ALLOY CD11U 11-Cadmium uranium Cd11U 1474.536865 ALLOY CD3AS2 3-Cadmium 2-arsenic As2Cd3 487.073212 -

Investigation of Iron Selenide Superconducting Thin Films Fabricated by Pulsed Laser Deposition
University of Wollongong Research Online University of Wollongong Thesis Collection 2017+ University of Wollongong Thesis Collections 2018 Investigation of Iron Selenide Superconducting Thin Films Fabricated by Pulsed Laser Deposition Wenbin Qiu University of Wollongong Follow this and additional works at: https://ro.uow.edu.au/theses1 University of Wollongong Copyright Warning You may print or download ONE copy of this document for the purpose of your own research or study. The University does not authorise you to copy, communicate or otherwise make available electronically to any other person any copyright material contained on this site. You are reminded of the following: This work is copyright. Apart from any use permitted under the Copyright Act 1968, no part of this work may be reproduced by any process, nor may any other exclusive right be exercised, without the permission of the author. Copyright owners are entitled to take legal action against persons who infringe their copyright. A reproduction of material that is protected by copyright may be a copyright infringement. A court may impose penalties and award damages in relation to offences and infringements relating to copyright material. Higher penalties may apply, and higher damages may be awarded, for offences and infringements involving the conversion of material into digital or electronic form. Unless otherwise indicated, the views expressed in this thesis are those of the author and do not necessarily represent the views of the University of Wollongong. Recommended Citation Qiu, Wenbin, Investigation of Iron Selenide Superconducting Thin Films Fabricated by Pulsed Laser Deposition, Doctor of Philosophy thesis, Institute for Superconducting and Electronic Materials, University of Wollongong, 2018. -
Standard X-Ray Diffraction Powder Patterns
E^l Admin. NBS MONOGRAPH 25—SECTION 5 Refecii^M not to be ^ferlrom the library. Standard X-ray Diffraction Powder Patterns ^\ / U.S. DEPARTMENT OF COMMERCE S NATIONAL BUREAU OF STANDARDS THE NATIONAL BUREAU OF STANDARDS The National Bureau of Standards^ provides measurement and technical information services essential to the efficiency and effectiveness of the work of the Nation's scientists and engineers. The Bureau serves also as a focal point in the Federal Government for assuring maximum application of the physical and engineering sciences to the advancement of technology in industry and commerce. To accomplish this mission, the Bureau is organized into three institutes covering broad program areas of research and services: THE INSTITUTE FOR BASIC STANDARDS . provides the central basis within the United States for a complete and consistent system of physical measurements, coordinates that system with the measurement systems of other nations, and furnishes essential services leading to accurate and uniform physical measurements throughout the Nation's scientific community, industry, and commerce. This Institute comprises a series of divisions, each serving a classical subject matter area: —Applied Mathematics—Electricity—Metrology—Mechanics—Heat—Atomic Physics—Physical Chemistry—Radiation Physics— -Laboratory Astrophysics^—Radio Standards Laboratory,^ which includes Radio Standards Physics and Radio Standards Engineering—Office of Standard Refer- ence Data. THE INSTITUTE FOR MATERIALS RESEARCH . conducts materials research and provides associated materials services including mainly reference materials and data on the properties of ma- terials. Beyond its direct interest to the Nation's scientists and engineers, this Institute yields services which are essential to the advancement of technology in industry and commerce. -

Thermoelectric Properties of the Heterostructural Alloy Sn Michael
Thermoelectric Properties of the Heterostructural Alloy Sn1−xCaxSe Michael Forkner Advisor: Dr. Janet Tate A thesis presented for the degree of Bachelor's of Science Department of Physics Oregon State University May 12th, 2017 Acknowledgments I would like to thank my PI, Dr. Janet Tate for allowing me to conduct my research in her laboratory. I would also like to thank graduate student Bethany Matthews for teaching me how to use the equipment, creating the samples, help with data anaylsis, and general help at all stages of this process. James Haggerty taught me how to use the Raman spectroscopy system and analyze the data. Dr. Aaron Holder and Dr. Stephan Lany at NREL created the theoretical material. Thank you to the OSU Physics Department for providing my physics education. This work was supported as part of the Center for the Next Generation of Materials by Design, an Energy Frontier Research Center funded by the U.S. Department of Energy, Office of Science. Contents 1 Introduction 1 1.1 Background . .1 1.2 Motivation . .1 1.3 SnSe and CaSe . .1 2 Theory 2 2.1 Metastability and Alloying . .2 2.2 Hall Effect . .3 2.3 Thermoelectric Effect . .4 2.4 Power Factor . .6 2.5 Raman Spectroscopy . .6 3 Methods 7 3.1 Thin Film Deposition . .7 3.2 Electrical Film Characterization . .7 3.2.1 Thermoelectric Effect . .7 3.2.2 Hall Effect . .8 3.3 Raman Characterization . .9 4 Results and Discussion 9 4.1 Seebeck Coefficient . .9 4.2 Carrier Density . 10 4.3 Resistivity . 12 4.4 Power Factor . -

Chapter 2 Test Bank the Components of Matter
Chapter 2 Test Bank The Components of Matter 1. Kaolinite, a clay mineral with the formula Al4Si4O10(OH)8, is used as a filler in slick-paper for magazines and as a raw material for ceramics. Analysis shows that 14.35 g of kaolinite contains 8.009 g of oxygen. Calculate the mass percent of oxygen in kaolinite. A. 1.792 mass % B. 24.80 mass % C. 30.81 mass % D. 34.12 mass % E. 55.81 mass % Accessibility: Keyboard Navigation Bloom's: 3. Apply Difficulty: Easy Gradable: automatic Subtopic: Atomic Theories Topic: Components of Matter 2. Compound 1 has a composition of 46.7 mass % of element A and 53.3 mass % of element B. A and B also form a second binary compound (compound 2). If the compositions of the two compounds are consistent with the law of multiple proportions, which of the following compositions could be that of compound 2? A. 23.4 mass % A 76.6 mass % B B. 30.4 mass % A 69.6 mass % B C. 33.3 mass % A 66.7 mass % B D. 53.3 mass % A 46.7 mass % B E. 73.3 mass % A 26.7 mass % B Accessibility: Keyboard Navigation Bloom's: 3. Apply Difficulty: Medium Gradable: automatic Subtopic: Atomic Theories Topic: Components of Matter 3. What are the approximate carbon:hydrogen mass ratios in methane (CH4) and ethyne (C2H2)? A. 1:4 and 1:1 B. 3:2 and 6:1 C. 3:1 and 12:1 D. 3:2 and 12:1 E. 3:1 and 6:1 Accessibility: Keyboard Navigation Bloom's: 3. -

Organic and Inorganic Selenium in Poultry: a Review
B-561 [1-8] Indian J. Anim. Res., AGRICULTURAL RESEARCH COMMUNICATION CENTRE Print ISSN:0367-6722 / Online ISSN:0976-0555 www.arccjournals.com/www.ijaronline.in Organic and inorganic selenium in poultry: A review Waseem Muhammad Zia1*, Anjum Khalique1, Saima Naveed1 and Jibran Hussain2 Department of Animal Nutrition, University of Veterinary and Animal Sciences, Faculty of Animal Production and Technology, Ravi Campus, Lahore-54000, Pakistan. Received: 04-07-2016 Accepted: 11-10-2016 ABSTRACT Selenium was believed to be toxic to animals, however, in 1957, selenium was reported as a dietary vital nutrient. Selenium is available in inorganic and organic forms. In 1974, the Food and Drug Administration (FDA) regulated the supplementation of selenium in poultry diets. In 1994, the National Research Council recognized selenium as a dietary essential nutrient for laying hens. The maximum allowed selenium addition level is 0.30 mg/kg. One of the most common supplements used is sodium selenite (SS), the inorganic selenium source. However, in 2000, the FDA approved the use of the organic source of selenium, Se-enriched yeast (SY) in poultry diets. Selenium has valuable effects on animal immune status, growth parameters, production and reproduction. Experimentally, it has been indicated that SY benefits more than that of SS due its more bioavailability. Selenium inclusion in food-animal diets has an extra nutritional advantage to human consumers of Se- enriched food-animal products. Key words: Se-enriched yeast, Sodium selenite, Nutrient utilization, Growth, Production performance, Egg quality, Functional foods, Hatching characteristics. INTRODUCTION Elementary selenium: It is stable and exists in modifications Selenium (Se) was discovered in 1817 and primary which are virtually biologically inactive, especially for its interest in the element was shown by glassmaking, pottery, poor resorption.