Appendix 4 Subtidal Survey March 2019
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Download PDF Version
MarLIN Marine Information Network Information on the species and habitats around the coasts and sea of the British Isles Lagoon cockle (Cerastoderma glaucum) MarLIN – Marine Life Information Network Biology and Sensitivity Key Information Review Nicola White 2002-07-15 A report from: The Marine Life Information Network, Marine Biological Association of the United Kingdom. Please note. This MarESA report is a dated version of the online review. Please refer to the website for the most up-to-date version [https://www.marlin.ac.uk/species/detail/1315]. All terms and the MarESA methodology are outlined on the website (https://www.marlin.ac.uk) This review can be cited as: White, N. 2002. Cerastoderma glaucum Lagoon cockle. In Tyler-Walters H. and Hiscock K. (eds) Marine Life Information Network: Biology and Sensitivity Key Information Reviews, [on-line]. Plymouth: Marine Biological Association of the United Kingdom. DOI https://dx.doi.org/10.17031/marlinsp.1315.1 The information (TEXT ONLY) provided by the Marine Life Information Network (MarLIN) is licensed under a Creative Commons Attribution-Non-Commercial-Share Alike 2.0 UK: England & Wales License. Note that images and other media featured on this page are each governed by their own terms and conditions and they may or may not be available for reuse. Permissions beyond the scope of this license are available here. Based on a work at www.marlin.ac.uk (page left blank) Date: 2002-07-15 Lagoon cockle (Cerastoderma glaucum) - Marine Life Information Network See online review for distribution map Three Cerastoderma glaucum with siphons extended. Distribution data supplied by the Ocean Photographer: Dennis R. -

Spatial Variability in Recruitment of an Infaunal Bivalve
Spatial Variability in Recruitment of an Infaunal Bivalve: Experimental Effects of Predator Exclusion on the Softshell Clam (Mya arenaria L.) along Three Tidal Estuaries in Southern Maine, USA Author(s): Brian F. Beal, Chad R. Coffin, Sara F. Randall, Clint A. Goodenow Jr., Kyle E. Pepperman, Bennett W. Ellis, Cody B. Jourdet and George C. Protopopescu Source: Journal of Shellfish Research, 37(1):1-27. Published By: National Shellfisheries Association https://doi.org/10.2983/035.037.0101 URL: http://www.bioone.org/doi/full/10.2983/035.037.0101 BioOne (www.bioone.org) is a nonprofit, online aggregation of core research in the biological, ecological, and environmental sciences. BioOne provides a sustainable online platform for over 170 journals and books published by nonprofit societies, associations, museums, institutions, and presses. Your use of this PDF, the BioOne Web site, and all posted and associated content indicates your acceptance of BioOne’s Terms of Use, available at www.bioone.org/page/terms_of_use. Usage of BioOne content is strictly limited to personal, educational, and non-commercial use. Commercial inquiries or rights and permissions requests should be directed to the individual publisher as copyright holder. BioOne sees sustainable scholarly publishing as an inherently collaborative enterprise connecting authors, nonprofit publishers, academic institutions, research libraries, and research funders in the common goal of maximizing access to critical research. Journal of Shellfish Research, Vol. 37, No. 1, 1–27, 2018. SPATIAL VARIABILITY IN RECRUITMENT OF AN INFAUNAL BIVALVE: EXPERIMENTAL EFFECTS OF PREDATOR EXCLUSION ON THE SOFTSHELL CLAM (MYA ARENARIA L.) ALONG THREE TIDAL ESTUARIES IN SOUTHERN MAINE, USA 1,2 3 2 3 BRIAN F. -

Outer Ards Modiolus Modiolus Report
R Assessment of Outer Ards Modiolus modiolus biogenic reefs against Special Area of Conservation (SAC) criteria JULY 2016 A report from the Fisheries and Aquatic Ecosystems Branch, Agri-food and Biosciences Institute to The Department of Agriculture, Environment and Rural Affairs (Northern Ireland) Document version control: Version Issue date Modifier Note Issued to and date 1.0 31/03/2016 AFBI-AC First draft for review DAERA & MS: 31/03/2016 1.1 19/05/2016 AFBI-AC Second draft for review DAERA & MS: 19/05/2016 1.2 19/07/2016 AFBI-AC Final draft for sign off following MS: 19/07/2016 receipt of comments 22/06/2016 1.3 19/07/2016 AFBI-AC Final version DAERA: 20/07/2016 Further information Dr. Annika Clements Seabed Habitat Mapping Project Leader Fisheries & Aquatic Ecosystems Branch Newforge Lane Belfast BT9 5PX Tel: +44(0)2890255153 Email: [email protected] DAERA Client Officer Joe Breen DAERA, Marine Conservation and Reporting Team Marine & Fisheries Division Portrush Coastal Zone 8 Bath Road PORTRUSH BT56 8AP Tel: +44(0)2870823600 (ext31) Email: [email protected] The GIS project “Outer_Ards_Modiolus_2016.mxd” should be available for use in conjunction with this report. Recommended citation: AFBI, 2016. Special Area of Conservation Designation Assessment of Outer Ards Modiolus modiolus Biogenic Reef. Report to the Department of Agriculture, Environment and Rural Affairs, Northern Ireland. Acknowledgements The author wishes to thank Adele Boyd and Matthew Service (AFBI) for data provision, James McArdle (AFBI), Katie Lilley (Ulster University placement student with AFBI) and Clara Alvarez Alonso (DAERA) and the master and crew of the R.V. -

TREATISE ONLINE Number 48
TREATISE ONLINE Number 48 Part N, Revised, Volume 1, Chapter 31: Illustrated Glossary of the Bivalvia Joseph G. Carter, Peter J. Harries, Nikolaus Malchus, André F. Sartori, Laurie C. Anderson, Rüdiger Bieler, Arthur E. Bogan, Eugene V. Coan, John C. W. Cope, Simon M. Cragg, José R. García-March, Jørgen Hylleberg, Patricia Kelley, Karl Kleemann, Jiří Kříž, Christopher McRoberts, Paula M. Mikkelsen, John Pojeta, Jr., Peter W. Skelton, Ilya Tëmkin, Thomas Yancey, and Alexandra Zieritz 2012 Lawrence, Kansas, USA ISSN 2153-4012 (online) paleo.ku.edu/treatiseonline PART N, REVISED, VOLUME 1, CHAPTER 31: ILLUSTRATED GLOSSARY OF THE BIVALVIA JOSEPH G. CARTER,1 PETER J. HARRIES,2 NIKOLAUS MALCHUS,3 ANDRÉ F. SARTORI,4 LAURIE C. ANDERSON,5 RÜDIGER BIELER,6 ARTHUR E. BOGAN,7 EUGENE V. COAN,8 JOHN C. W. COPE,9 SIMON M. CRAgg,10 JOSÉ R. GARCÍA-MARCH,11 JØRGEN HYLLEBERG,12 PATRICIA KELLEY,13 KARL KLEEMAnn,14 JIřÍ KřÍž,15 CHRISTOPHER MCROBERTS,16 PAULA M. MIKKELSEN,17 JOHN POJETA, JR.,18 PETER W. SKELTON,19 ILYA TËMKIN,20 THOMAS YAncEY,21 and ALEXANDRA ZIERITZ22 [1University of North Carolina, Chapel Hill, USA, [email protected]; 2University of South Florida, Tampa, USA, [email protected], [email protected]; 3Institut Català de Paleontologia (ICP), Catalunya, Spain, [email protected], [email protected]; 4Field Museum of Natural History, Chicago, USA, [email protected]; 5South Dakota School of Mines and Technology, Rapid City, [email protected]; 6Field Museum of Natural History, Chicago, USA, [email protected]; 7North -

HETA ROUSI: Zoobenthos As Indicators of Marine Habitats in the Northern Baltic
Heta Rousi Zoobenthos as indicators of marine Heta Rousi | habitats in the northern Baltic Sea of marine as indicators habitats in the northernZoobenthos Baltic Sea Heta Rousi This thesis describes how physical and chemical environmental variables impact zoobenthic species distribution in the northern Baltic Sea and how dis- Zoobenthos as indicators of marine tinct zoobenthic species indicate different marine benthic habitats. The thesis inspects the effects of habitats in the northern Baltic Sea depth, sediment type, temperature, salinity, oxy- gen, nutrients as well as topographical and geo- logical factors on zoobenthos on small and large temporal and spatial scales. | 2020 ISBN 978-952-12-3944-1 Heta Rousi Född 1979 Studier och examina Magister vid Helsingfors Universitet 2006 Licentiat vid Åbo Akademi 2013 Doktorsexamen vid Åbo Akademi 2020 Institutionen för miljö- och marinbiologi, Åbo Akademi ZOOBENTHOS AS INDICATORS OF MARINE HABITATS IN THE NORTHERN BALTIC SEA HETA ROUSI Environmental and Marine Biology Faculty of Science and Engineering Åbo Akademi University Finland, 2020 SUPERVISED BY PRE-EXAMINED BY Professor Erik Bonsdorff Research Professor (Supervisor & Examiner) Markku Viitasalo Åbo Akademi University Finnish Environment Institute Faculty of Science and Engineering Sustainable Use of the Marine Areas Environmental and Marine Biology Latokartanonkaari 11 Artillerigatan 6 00790 Helsinki 20520 Åbo Finland Finland Professor Emeritus Ilppo Vuorinen CO-SUPERVISOR University of Turku Adjunct Professor Faculty of Science and Engineering Samuli Korpinen Itäinen Pitkäkatu 4 Finnish Environment Institute 20520 Turku Marine Management Finland Latokartanonkaari 11 00790 Helsinki FACULTY OPPONENT Finland Associate Professor Urszula Janas SUPERVISING AT THE University of Gdansk LICENCIATE PHASE Institute of Oceanography Assistant Professor Al. -
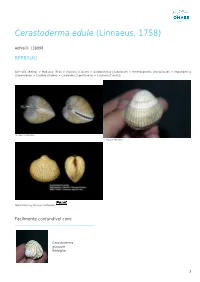
Cerastoderma Edule (Linnaeus, 1758)
Cerastoderma edule (Linnaeus, 1758) AphiaID: 138998 BERBIGÃO Animalia (Reino) > Mollusca (Filo) > Bivalvia (Classe) > Autobranchia (Subclasse) > Heteroconchia (Infraclasse) > Imparidentia (Superordem) > Cardiida (Ordem) > Cardioidea (Superfamilia) > Cardiidae (Familia) © Vasco Ferreira © Vasco Ferreira Natural History Museum Rotterdam Facilmente confundível com: Cerastoderma glaucum Berbigão 1 Estatuto de Conservação Sinónimos Cardium belgicum De Malzine, 1867 Cardium crenulatum Lamarck, 1819 Cardium edule Linnaeus, 1758 Cardium edule burchanae Girscher, 1938 Cardium edule var. batesoni Bucquoy, Dautzenberg & Dollfus, 1892 Cardium edule var. loppensi Mars, 1951 Cardium edule var. maculata Dautzenberg, 1890 Cardium edule var. major Bucquoy, Dautzenberg & Dollfus, 1892 Cardium edule var. mareotica Pallary, 1912 Cardium edule var. regularis Pallary, 1900 Cardium edule var. sibenicensis Brusina, 1870 Cardium mercatorium Coen, 1915 Cardium nunninkae Lucas, 1984 Cardium obtritum Locard, 1886 Cardium quadrarium Reeve, 1845 Cardium vulgare da Costa, 1778 Cardium vulgatum Tryon, 1872 Cerastoderma edule var. sinicola Lacourt, 1974 Cerastoderma nunninkae Lucas, 1984 Referências basis of record Gofas, S.; Le Renard, J.; Bouchet, P. (2001). Mollusca. in: Costello, M.J. et al. (eds), European Register of Marine Species: a check-list of the marine species in Europe and a bibliography of guides to their identification. Patrimoines Naturels. 50: 180-213. [details] additional source Poorten, J.J. ter, 2005. Outline of a systematic index – Recent Cardiidae (Lamarck, 1809). VISAYA net. (Updated 2009 for WoRMS), available online at http://www.conchology.be/en/shelltopics/visaya-net/date.php?year=2005 [details] 2 ecology source Coscia, I., P.E. Robins, J.S. Porter, S.K. Malham & J.E. Ironside. (2013). Modelled larval dispersal and measured gene flow: seascape genetics of the common cockle Cerastoderma edule in the southern Irish Sea. -

Macrobenthic Assemblage Structure and Distribution at the Boojagh Marine National Park, Southern Caspian Sea, Iran
Iranian Journal of Fisheries Sciences 19(2) 748-767 2020 DOI: 10.22092/ijfs.2020.120829 Macrobenthic assemblage structure and distribution at the Boojagh Marine National Park, Southern Caspian Sea, Iran. Bahrebar S.¹; Negarestan H.²*; Maghsoudlo A.³; Danehkar A.4 Received: December 2016 Accepted: Febtuary 2017 Abstract Although macrobenthic assemblages are considered as major players in many ecosystems around the world, the ecology of Caspian Sea macrobenthos is currently understudied. This study describes the species composition and quantitative distribution of macrobenthos in the southern Caspian Sea and relates the distribution to seasonal changes at three depths (1, 5 and 10 meters) on the Boojagh Marine National Park (BMNP) coast in the southern Caspian Sea between the summers of 2015 and 2016. To investigate the distribution of macrobenthos in BMNP, the data of 450 samples were analyzed. In this study sixteen species were identified: Cerastoderma glaucum, Mytilaster lineatus, Pyrgula grimmi, Anisus kolesnikovi, Stenogammarus carausui, Paraniphargoides motasi, Onisimus caspius, Pterocuma pectinatum, Pterocuma sowinskyi, Pseudocuma (Stenocuma) gracile, Nais sp., Hypania invalida, Manayunkia caspica, Streblospio gynobranchiata, Hediste diversicolor, Amphibalanus improvisus. Among them, the non-indigenous C. glaucum was the dominant species, accounting for 27% of the total abundance and in descending order P. grimmi with 14.4%, A. improvisus with 8.7%, M. lineatus with 7.9%, Nais sp. with 7.5%, N. carausui with 5.2%, P. motasi with 5%, S. gynobranchiata with 4.5%, H. invalida with 5%, M. Caspica with 3.1%, P. sowinskyi with 2.5%, O. caspius with 2.4%, A. kolesnikovi and H. diversicolor with 1.8%, S. -

Limecola Balthica
Phylum: Mollusca Macoma balthica Class: Bivalvia, Heterodonta, Euheterodonta Order: Imparidentia, Cardiida Family: Tellinoidea, Tellinidae, Macominae Taxonomy: Originally described as a mem- Description ber of the genus Tellina, Macoma balthica Size: Individuals averaging 30–35 mm in was the name of the Atlantic species. Our length (Oldroyd 1924), but usually under 30 west coast clam was originally called M. in- mm (Coan 1971) and rarely more than 45 mm conspicua (Broderip and Sowerby 1829), but (Coan 1971; Cardoso et al. 2003). Smallest they are now generally considered to be the adults are 2 mm (Caddy 1969). Body propor- same species (e.g., Vassallo, 1969, 1971; tions are generally 27 in length, 22 in height, Haderlie and Abbott 1980). An extensive and 11 mm in diameter (Oldroyd 1924). The taxonomic history has yielded many sy- illustrated specimen (from Coos Bay) is 17.5 nonyms for M. balthica. Some ambiguity mm long. exists whether individuals from the southern- Color: Distinct color is reddish, pale rose or most reaches of the distribution on east and white and is sometimes bluish or yellow west sides of the Atlantic should be conside- (Oldroyd 1924; see Plate 17, Kozloff 1993). red the same species (Beukema and Coos Bay specimens are usually pink inside Meehan 1985) and some researchers (e.g., and out, but individuals from British Columbia, Meehan 1985; Kamermans et al. 1990; Lut- Canada can have pink or yellow interiors tikhuizen et al. 2012; Sanier et al. 2015) (Quayle 1970). consider these allopatric populations to be General Morphology: Bivalve mollusks are subspecies (eastern Atlantic Macoma balthi- bilaterally symmetrical with two lateral valves ca balthica and western Atlantic Macoma or shells that are hinged dorsally and sur- balthica rubra) that have been reproductively round a mantle, head, foot and viscera (see isolated for 2–3.5 million years (Luttikhuizen Plate 393B, Coan and Valentich-Scott 2007). -

Macoma Inquinata Class: Bivalvia, Heterodonta, Euheterodonta
Phylum: Mollusca Macoma inquinata Class: Bivalvia, Heterodonta, Euheterodonta Order: Imparidentia, Cardiida Irus clam Family: Tellinoidea, Tellinidae, Macominae Taxonomy: Macoma balthica, M. nasuta (see Plate 393B, Coan and Valentich-Scott and M. inquinata were all originally de- 2007). Among the bivalves, the Heterodonta scribed as members of the genus Tellina. are characterized by ctenidia (or gills) that Tellina inquinata and T. irus, initially de- are eulamellibranchiate, fused mantle margins scribed as different species (the former with and the presence of long siphons. Veneroid eastern Pacific distribution, the latter with bivalves have well-developed hinge teeth and western), were synonymized in the genus members of the family Tellinidae have short Heteromacoma. Later, this synonymization lateral hinge teeth (when present – see Pos- was reversed based on characters of shell sible Misidentifications), shells with external morphology and Macoma inquinata striations or ribs, and deep pallial sinuses (previously, and confusingly, called M. irus) (Coan and Valentich-Scott 2007). When was deemed a member of the genus Maco- holding closed shell in both hands with the ma, with an eastern Pacific distribution while hinged area up and the ligaments toward you, H. irus, remained a Heteromacoma, with a the right valve is in the right hand (Fig. 4) the western Pacific (see Keen 1962; Coan (Keen and Coan 1974). 1971). Thus, known synonyms for M. in- Body: quinata include T. inquinata as well as M. Color: irus. Subspecific designations are also Interior: Ligament is long, strong, nar- sometimes seen (e.g. Macoma heteromaco- row, and prominent (Figs. 1, 4). It is not ma inquinata, Kabat and O’Foighil 1987). -

Chemical & Biological Survey at Baltray October 2016
Chemical & Biological Survey at Baltray October 2016 Produced by AQUAFACT International Services Ltd On behalf of Premier Periclase Ltd. December 2016 For inspection purposes only. Consent of copyright owner required for any other use. AQUAFACT INTERNATIONAL SERVICES LTD., 12 KILKERRIN PARK, GALWAY . www.aquafact.ie [email protected] tel +353 (0) 91 756812 EPA Export 29-03-2017:23:50:59 Table of Contents 1. Introduction ................................................................................................... 1 2. Summary ........................................................................................................ 2 3. Materials & Methods ..................................................................................... 2 3.1. Biological Sampling .............................................................................................. 3 3.2. Sediment Sampling .............................................................................................. 4 3.3. Water Sampling ................................................................................................... 4 4. Results ........................................................................................................... 5 4.1. Biological Results ................................................................................................. 5 4.2. Sediment Results ................................................................................................. 6 4.3. Water Results ..................................................................................................... -

Mulinia Lateralis (Say, 1822) in Europe J
Craeymeersch et al. Marine Biodiversity Records (2019) 12:5 https://doi.org/10.1186/s41200-019-0164-7 MARINE RECORD Open Access First records of the dwarf surf clam Mulinia lateralis (Say, 1822) in Europe J. A. Craeymeersch1* , M. A. Faasse2,3, H. Gheerardyn2, K. Troost1, R. Nijland5 , A. Engelberts4 , K. J. Perdon1, D. van den Ende1 and J. van Zwol1 Abstract This paper reports the first records of the dwarf surf clam Mulinia lateralis (Say, 1822) outside its native area, which is the western Atlantic Ocean, ranging from theGulfofStLawrencetotheGulfofMexico.In2017 and 2018 specimens were found in the Dutch coastal waters (North Sea), in the Wadden Sea and in the Westerschelde estuary, in densities of up to almost 6000 individuals per square meter. In view of its ecology and distributional range in the native area M. lateralis has the potential to become an invasive species. Its ability to quickly colonize defaunated areas, its high fecundity and short generation time, its tolerance for anoxia and temperature extremes and its efficient exploitation of the high concentrations of phytoplankton and natural seston at the sediment-water interface may bring it into competition with native species for food and space. Keywords: Mulinia lateralis, Bivalvia, Marine, North Sea, Invasive, Competition Introduction shipping (Port of Rotterdam) and aquaculture (Oos- For at least half a century, the process and the envir- terschelde estuary) activities in the North Sea region onmental, economic and social impacts of invasions (see also Wolff 2005). The most successful taxa re- of non-indigenous species are a focus of ecological garding introduction and immigration are poly- research (Elton 1958; Rilov and Crooks 2009; chaetes, bivalves and amphipods (Reise et al. -

A Comparative Study Onspecies Diversity of Marine Bivalves From
Dagon University Commemoration of 25th Anniversary Silver Jubilee Research Journal 2019, Vol.9, No.2 231 A Comparative Study onSpecies Diversity of Marine Bivalves from Maungmagan and KyaukKaMaunkCoastals KhinMyo Myat1,LattLatt Htwe2,Kathy Khine3, Wint War Nway4, Nay Htet Lin5, Win Thit Oo6 ABSTRACT In the present study, survey of marine bivalves for species diversity was done along two coastals; Maungmagan and KyaukKaMaunkcoastals, of Tanintharyi Region. Marine bivalves were collected during low tides from intertidal region and shallow coastal waters from December 2017 to November 2018. Total 30 species of bivalves belonging to 19 genera, 10families and 6orders were recorded from Maungmakan coast and KyaukKaMaunkcoastals. Bivalves belonging to families Arcidae, Ostreidae, Cardiidae, Glymeridae, Mytilidae, Limidae, Semelidae, Tellinidae,Lucinidaeand Veneridae were recorded during the study. Number of species of bivalves distributed in each family revolves that 6 species to Arcidae, 5 species belongs to family Veneridae and Semelidae4 species to Ostreidae, 3 species to Cardiida, LucinidaeandTellinidae to 2 species.1 species each were reported from families Glymeridae, Mytilidae, Limidae,. Maximum species diversity of bivalves is recorded from KyaukKaMaunk coastal. The bivalves at Maungmagan coastal result from related establishments, sedimentation, disposal of domestic sewage, industrial wastes, habitat loss and tourism. This study reveals that bivalves from Maungmagan coastal are facing threat due to industrial pollution. INTRODUCTION The bivalvia is the second most species class in the phylum Mollusca. Bivalves are distinctive within the Mollusca.A bivalve shell is part of the body, the exoskeleton or shell, of a bivalvemolluck,Tabugo and Gopalsamy(2013). Bivalves by definition possess two shells or valves, a "right valve" and a "left valve", that are joined by a ligament , Hamli (2012).This exoskeleton serves not only for muscle attachment, but also for protection from predators and from mechanical damage.