Limecola Balthica
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Seasonal Variation in the Occurrence of Planktic Bivalve Larvae in the Schleswig-Holstein Wadden Sea
HELGOLdkNDER MEERESUNTERSUCHUNGEN Helgolander Meeresunters. 51, 23-39 {1997} Seasonal variation in the occurrence of planktic bivalve larvae in the Schleswig-Holstein Wadden Sea Andrea Pulfrich* Institut ffir Meereskunde; Dfisternbrooker Weg 20, 24105 Kiel, Germany ABSTRACT: In the late 1980s, recruitment failures of the mussel Mytilus edulis led to economic pro- blems in the mussel fishing and cultivation industries of northwestern Europe. As part of a collabo- rative study to gain a better understanding of the mechanisms affecting recruitment processes of mussels, plankton samples were collected regularly over a four-year period (t990-I993) from three stations in the Schleswig-Holstein Wadden Sea. The bivalve component of the plankton was domi- nated by the Solenidae, which was almost exclusively represented by Ensis americanus [= directus). NI. eduhs was the second most abundant species. Abundances of mussel larvae peaked 2 to 4 weeks after spawning maxima in the adult populations. Although variations in timing and amplitude of the tota~ [arvaL densities occurred, annua[ abuadances o[M. edulis larvae remained stable during the study period, and regional abundance differences were insignificant. A close relaUonsh~p was found between peaks in larval abundance and phytoplankton blooms. Differences in larval concentrations in the ebb and the flow currents were insignificant. Planktic mussel larvae measured between 200 }am and 300 ~tm, and successive cohorts were recognizable in the majority of samples. Most lar- vae were found to originate from local stocks, although imports from outside the area do occur. INTRODUCTION Successive years of failing spatfall and recruitment of the edible mussel Mytilus edu- lis L. (Bivalvia) on the northwestern European coast during the late 1980s caused sub- stantial production Losses in the mussel fishing and cultivation industries. -

Download PDF Version
MarLIN Marine Information Network Information on the species and habitats around the coasts and sea of the British Isles Lagoon cockle (Cerastoderma glaucum) MarLIN – Marine Life Information Network Biology and Sensitivity Key Information Review Nicola White 2002-07-15 A report from: The Marine Life Information Network, Marine Biological Association of the United Kingdom. Please note. This MarESA report is a dated version of the online review. Please refer to the website for the most up-to-date version [https://www.marlin.ac.uk/species/detail/1315]. All terms and the MarESA methodology are outlined on the website (https://www.marlin.ac.uk) This review can be cited as: White, N. 2002. Cerastoderma glaucum Lagoon cockle. In Tyler-Walters H. and Hiscock K. (eds) Marine Life Information Network: Biology and Sensitivity Key Information Reviews, [on-line]. Plymouth: Marine Biological Association of the United Kingdom. DOI https://dx.doi.org/10.17031/marlinsp.1315.1 The information (TEXT ONLY) provided by the Marine Life Information Network (MarLIN) is licensed under a Creative Commons Attribution-Non-Commercial-Share Alike 2.0 UK: England & Wales License. Note that images and other media featured on this page are each governed by their own terms and conditions and they may or may not be available for reuse. Permissions beyond the scope of this license are available here. Based on a work at www.marlin.ac.uk (page left blank) Date: 2002-07-15 Lagoon cockle (Cerastoderma glaucum) - Marine Life Information Network See online review for distribution map Three Cerastoderma glaucum with siphons extended. Distribution data supplied by the Ocean Photographer: Dennis R. -

Spatial Variability in Recruitment of an Infaunal Bivalve
Spatial Variability in Recruitment of an Infaunal Bivalve: Experimental Effects of Predator Exclusion on the Softshell Clam (Mya arenaria L.) along Three Tidal Estuaries in Southern Maine, USA Author(s): Brian F. Beal, Chad R. Coffin, Sara F. Randall, Clint A. Goodenow Jr., Kyle E. Pepperman, Bennett W. Ellis, Cody B. Jourdet and George C. Protopopescu Source: Journal of Shellfish Research, 37(1):1-27. Published By: National Shellfisheries Association https://doi.org/10.2983/035.037.0101 URL: http://www.bioone.org/doi/full/10.2983/035.037.0101 BioOne (www.bioone.org) is a nonprofit, online aggregation of core research in the biological, ecological, and environmental sciences. BioOne provides a sustainable online platform for over 170 journals and books published by nonprofit societies, associations, museums, institutions, and presses. Your use of this PDF, the BioOne Web site, and all posted and associated content indicates your acceptance of BioOne’s Terms of Use, available at www.bioone.org/page/terms_of_use. Usage of BioOne content is strictly limited to personal, educational, and non-commercial use. Commercial inquiries or rights and permissions requests should be directed to the individual publisher as copyright holder. BioOne sees sustainable scholarly publishing as an inherently collaborative enterprise connecting authors, nonprofit publishers, academic institutions, research libraries, and research funders in the common goal of maximizing access to critical research. Journal of Shellfish Research, Vol. 37, No. 1, 1–27, 2018. SPATIAL VARIABILITY IN RECRUITMENT OF AN INFAUNAL BIVALVE: EXPERIMENTAL EFFECTS OF PREDATOR EXCLUSION ON THE SOFTSHELL CLAM (MYA ARENARIA L.) ALONG THREE TIDAL ESTUARIES IN SOUTHERN MAINE, USA 1,2 3 2 3 BRIAN F. -

Processing of 13C-Labelled Phytoplankton in a Fine-Grained Sandy-Shelf Sediment (North Sea): Relative Importance of Different Macrofauna Species
MARINE ECOLOGY PROGRESS SERIES Vol. 297: 61–70, 2005 Published August 1 Mar Ecol Prog Ser Processing of 13C-labelled phytoplankton in a fine-grained sandy-shelf sediment (North Sea): relative importance of different macrofauna species Anja Kamp1, 2,*, Ursula Witte1, 3 1Max Planck Institute for Marine Microbiology, Celsiusstr. 1, 28359 Bremen, Germany 2Present address: Institute for Microbiology, University of Hannover, Schneiderberg 50, 30167 Hannover, Germany 3Present address: Oceanlab, University of Aberdeen, Newburgh, Aberdeen AB41 6AA, UK ABSTRACT: On-board and in situ experiments with 13C-labelled diatoms were carried out to inves- tigate the processing of algal carbon by the macrofauna community of a fine sandy-shelf site in the southern German Bight (North Sea). The time series (12, 30, 32 and 132 h incubations) was supple- mented by additional laboratory experiments on the role of the dominant macrofauna organism, the bivalve Fabulina fabula (Bivalvia: Tellinidae), for particulate organic matter subduction to deeper sediment layers. The specific uptake of algal 13C by macrofauna organisms was visible after 12 h and constantly increased during the incubation periods. F. fabula, a facultative (surface) deposit- and suspension-feeder, Lanice conchilega (Polychaeta: Terebellidae), a suspension-feeder and the (sur- face) deposit-feeder Echinocardium cordatum (Echinodermata: Spatangidae) were responsible for the majority of macrofaunal carbon processing. Predatory macrofauna organisms like Nephtys spp. (Polychaeta: Nephtyidae) also quickly became labelled. The rapid subduction of fresh organic matter by F. fabula down to ca. 4 to 7 cm sediment depth could be demonstrated, and it is suggested that entrainment by macrofauna in this fine-grained sand is much more efficient than advective transport. -

Outer Ards Modiolus Modiolus Report
R Assessment of Outer Ards Modiolus modiolus biogenic reefs against Special Area of Conservation (SAC) criteria JULY 2016 A report from the Fisheries and Aquatic Ecosystems Branch, Agri-food and Biosciences Institute to The Department of Agriculture, Environment and Rural Affairs (Northern Ireland) Document version control: Version Issue date Modifier Note Issued to and date 1.0 31/03/2016 AFBI-AC First draft for review DAERA & MS: 31/03/2016 1.1 19/05/2016 AFBI-AC Second draft for review DAERA & MS: 19/05/2016 1.2 19/07/2016 AFBI-AC Final draft for sign off following MS: 19/07/2016 receipt of comments 22/06/2016 1.3 19/07/2016 AFBI-AC Final version DAERA: 20/07/2016 Further information Dr. Annika Clements Seabed Habitat Mapping Project Leader Fisheries & Aquatic Ecosystems Branch Newforge Lane Belfast BT9 5PX Tel: +44(0)2890255153 Email: [email protected] DAERA Client Officer Joe Breen DAERA, Marine Conservation and Reporting Team Marine & Fisheries Division Portrush Coastal Zone 8 Bath Road PORTRUSH BT56 8AP Tel: +44(0)2870823600 (ext31) Email: [email protected] The GIS project “Outer_Ards_Modiolus_2016.mxd” should be available for use in conjunction with this report. Recommended citation: AFBI, 2016. Special Area of Conservation Designation Assessment of Outer Ards Modiolus modiolus Biogenic Reef. Report to the Department of Agriculture, Environment and Rural Affairs, Northern Ireland. Acknowledgements The author wishes to thank Adele Boyd and Matthew Service (AFBI) for data provision, James McArdle (AFBI), Katie Lilley (Ulster University placement student with AFBI) and Clara Alvarez Alonso (DAERA) and the master and crew of the R.V. -

Distribution, Abundance, and Diversity of Epifaunal Benthic Organisms in Alitak and Ugak Bays, Kodiak Island, Alaska
DISTRIBUTION, ABUNDANCE, AND DIVERSITY OF EPIFAUNAL BENTHIC ORGANISMS IN ALITAK AND UGAK BAYS, KODIAK ISLAND, ALASKA by Howard M. Feder and Stephen C. Jewett Institute of Marine Science University of Alaska Fairbanks, Alaska 99701 Final Report Outer Continental Shelf Environmental Assessment Program Research Unit 517 October 1977 279 We thank the following for assistance during this study: the crew of the MV Big Valley; Pete Jackson and James Blackburn of the Alaska Department of Fish and Game, Kodiak, for their assistance in a cooperative benthic trawl study; and University of Alaska Institute of Marine Science personnel Rosemary Hobson for assistance in data processing, Max Hoberg for shipboard assistance, and Nora Foster for taxonomic assistance. This study was funded by the Bureau of Land Management, Department of the Interior, through an interagency agreement with the National Oceanic and Atmospheric Administration, Department of Commerce, as part of the Alaska Outer Continental Shelf Environment Assessment Program (OCSEAP). SUMMARY OF OBJECTIVES, CONCLUSIONS, AND IMPLICATIONS WITH RESPECT TO OCS OIL AND GAS DEVELOPMENT Little is known about the biology of the invertebrate components of the shallow, nearshore benthos of the bays of Kodiak Island, and yet these components may be the ones most significantly affected by the impact of oil derived from offshore petroleum operations. Baseline information on species composition is essential before industrial activities take place in waters adjacent to Kodiak Island. It was the intent of this investigation to collect information on the composition, distribution, and biology of the epifaunal invertebrate components of two bays of Kodiak Island. The specific objectives of this study were: 1) A qualitative inventory of dominant benthic invertebrate epifaunal species within two study sites (Alitak and Ugak bays). -

TREATISE ONLINE Number 48
TREATISE ONLINE Number 48 Part N, Revised, Volume 1, Chapter 31: Illustrated Glossary of the Bivalvia Joseph G. Carter, Peter J. Harries, Nikolaus Malchus, André F. Sartori, Laurie C. Anderson, Rüdiger Bieler, Arthur E. Bogan, Eugene V. Coan, John C. W. Cope, Simon M. Cragg, José R. García-March, Jørgen Hylleberg, Patricia Kelley, Karl Kleemann, Jiří Kříž, Christopher McRoberts, Paula M. Mikkelsen, John Pojeta, Jr., Peter W. Skelton, Ilya Tëmkin, Thomas Yancey, and Alexandra Zieritz 2012 Lawrence, Kansas, USA ISSN 2153-4012 (online) paleo.ku.edu/treatiseonline PART N, REVISED, VOLUME 1, CHAPTER 31: ILLUSTRATED GLOSSARY OF THE BIVALVIA JOSEPH G. CARTER,1 PETER J. HARRIES,2 NIKOLAUS MALCHUS,3 ANDRÉ F. SARTORI,4 LAURIE C. ANDERSON,5 RÜDIGER BIELER,6 ARTHUR E. BOGAN,7 EUGENE V. COAN,8 JOHN C. W. COPE,9 SIMON M. CRAgg,10 JOSÉ R. GARCÍA-MARCH,11 JØRGEN HYLLEBERG,12 PATRICIA KELLEY,13 KARL KLEEMAnn,14 JIřÍ KřÍž,15 CHRISTOPHER MCROBERTS,16 PAULA M. MIKKELSEN,17 JOHN POJETA, JR.,18 PETER W. SKELTON,19 ILYA TËMKIN,20 THOMAS YAncEY,21 and ALEXANDRA ZIERITZ22 [1University of North Carolina, Chapel Hill, USA, [email protected]; 2University of South Florida, Tampa, USA, [email protected], [email protected]; 3Institut Català de Paleontologia (ICP), Catalunya, Spain, [email protected], [email protected]; 4Field Museum of Natural History, Chicago, USA, [email protected]; 5South Dakota School of Mines and Technology, Rapid City, [email protected]; 6Field Museum of Natural History, Chicago, USA, [email protected]; 7North -

Cellular Level Response of the Bivalve Limecola Balthica To
Science of the Total Environment 794 (2021) 148593 Contents lists available at ScienceDirect Science of the Total Environment journal homepage: www.elsevier.com/locate/scitotenv Cellular level response of the bivalve Limecola balthica to seawater acidification due to potential CO2 leakage from a sub-seabed storage site in the southern Baltic Sea: TiTank experiment at representative hydrostatic pressure Adam Sokołowski a,1, Justyna Świeżak a,⁎,1, Anna Hallmann b, Anders J. Olsen c, Marcelina Ziółkowska a, Ida Beathe Øverjordet d, Trond Nordtug d, Dag Altin e, Daniel Franklin Krause d, Iurgi Salaberria c, Katarzyna Smolarz a a University of Gdańsk, Faculty of Oceanography and Geography, Institute of Oceanography, Al. Piłsudskiego 46, 81-378 Gdynia, Poland b Medical University of Gdańsk, Department of Pharmaceutical Biochemistry, Dębinki 1, 80-211 Gdańsk, Poland c Norwegian University of Science and Technology, NO-7491 Trondheim, Norway d SINTEF Ocean AS, Brattorkaia 17C, NO-7465 Trondheim, Norway e Altins Biotrix, Finn Bergs veg 3, 7022 Trondheim, Norway HIGHLIGHTS GRAPHICAL ABSTRACT • Cellular level responses of L. balthica to acidification caused by CO2 was tested at 9 ATM pressure. • The bivalve is tolerant to medium-term severe environmental hypercapnia. • Seawater pH 7.0 induced effects on rad- ical defence mechanisms (GPx, GST, CAT). • pH 6.3 caused increased cellular oxida- tive stress (MDA) and detoxification (tGSH). article info abstract Article history: Understanding of biological responses of marine fauna to seawater acidification due to potential CO2 leakage Received 7 April 2021 from sub-seabed storage sites has improved recently, providing support to CCS environmental risk assessment. Received in revised form 15 June 2021 Physiological responses of benthic organisms to ambient hypercapnia have been previously investigated but Accepted 17 June 2021 rarely at the cellular level, particularly in areas of less common geochemical and ecological conditions such as Available online 24 June 2021 brackish water and/or reduced oxygen levels. -

HETA ROUSI: Zoobenthos As Indicators of Marine Habitats in the Northern Baltic
Heta Rousi Zoobenthos as indicators of marine Heta Rousi | habitats in the northern Baltic Sea of marine as indicators habitats in the northernZoobenthos Baltic Sea Heta Rousi This thesis describes how physical and chemical environmental variables impact zoobenthic species distribution in the northern Baltic Sea and how dis- Zoobenthos as indicators of marine tinct zoobenthic species indicate different marine benthic habitats. The thesis inspects the effects of habitats in the northern Baltic Sea depth, sediment type, temperature, salinity, oxy- gen, nutrients as well as topographical and geo- logical factors on zoobenthos on small and large temporal and spatial scales. | 2020 ISBN 978-952-12-3944-1 Heta Rousi Född 1979 Studier och examina Magister vid Helsingfors Universitet 2006 Licentiat vid Åbo Akademi 2013 Doktorsexamen vid Åbo Akademi 2020 Institutionen för miljö- och marinbiologi, Åbo Akademi ZOOBENTHOS AS INDICATORS OF MARINE HABITATS IN THE NORTHERN BALTIC SEA HETA ROUSI Environmental and Marine Biology Faculty of Science and Engineering Åbo Akademi University Finland, 2020 SUPERVISED BY PRE-EXAMINED BY Professor Erik Bonsdorff Research Professor (Supervisor & Examiner) Markku Viitasalo Åbo Akademi University Finnish Environment Institute Faculty of Science and Engineering Sustainable Use of the Marine Areas Environmental and Marine Biology Latokartanonkaari 11 Artillerigatan 6 00790 Helsinki 20520 Åbo Finland Finland Professor Emeritus Ilppo Vuorinen CO-SUPERVISOR University of Turku Adjunct Professor Faculty of Science and Engineering Samuli Korpinen Itäinen Pitkäkatu 4 Finnish Environment Institute 20520 Turku Marine Management Finland Latokartanonkaari 11 00790 Helsinki FACULTY OPPONENT Finland Associate Professor Urszula Janas SUPERVISING AT THE University of Gdansk LICENCIATE PHASE Institute of Oceanography Assistant Professor Al. -
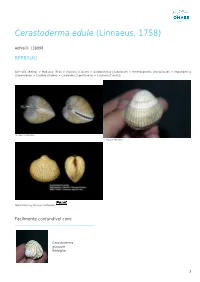
Cerastoderma Edule (Linnaeus, 1758)
Cerastoderma edule (Linnaeus, 1758) AphiaID: 138998 BERBIGÃO Animalia (Reino) > Mollusca (Filo) > Bivalvia (Classe) > Autobranchia (Subclasse) > Heteroconchia (Infraclasse) > Imparidentia (Superordem) > Cardiida (Ordem) > Cardioidea (Superfamilia) > Cardiidae (Familia) © Vasco Ferreira © Vasco Ferreira Natural History Museum Rotterdam Facilmente confundível com: Cerastoderma glaucum Berbigão 1 Estatuto de Conservação Sinónimos Cardium belgicum De Malzine, 1867 Cardium crenulatum Lamarck, 1819 Cardium edule Linnaeus, 1758 Cardium edule burchanae Girscher, 1938 Cardium edule var. batesoni Bucquoy, Dautzenberg & Dollfus, 1892 Cardium edule var. loppensi Mars, 1951 Cardium edule var. maculata Dautzenberg, 1890 Cardium edule var. major Bucquoy, Dautzenberg & Dollfus, 1892 Cardium edule var. mareotica Pallary, 1912 Cardium edule var. regularis Pallary, 1900 Cardium edule var. sibenicensis Brusina, 1870 Cardium mercatorium Coen, 1915 Cardium nunninkae Lucas, 1984 Cardium obtritum Locard, 1886 Cardium quadrarium Reeve, 1845 Cardium vulgare da Costa, 1778 Cardium vulgatum Tryon, 1872 Cerastoderma edule var. sinicola Lacourt, 1974 Cerastoderma nunninkae Lucas, 1984 Referências basis of record Gofas, S.; Le Renard, J.; Bouchet, P. (2001). Mollusca. in: Costello, M.J. et al. (eds), European Register of Marine Species: a check-list of the marine species in Europe and a bibliography of guides to their identification. Patrimoines Naturels. 50: 180-213. [details] additional source Poorten, J.J. ter, 2005. Outline of a systematic index – Recent Cardiidae (Lamarck, 1809). VISAYA net. (Updated 2009 for WoRMS), available online at http://www.conchology.be/en/shelltopics/visaya-net/date.php?year=2005 [details] 2 ecology source Coscia, I., P.E. Robins, J.S. Porter, S.K. Malham & J.E. Ironside. (2013). Modelled larval dispersal and measured gene flow: seascape genetics of the common cockle Cerastoderma edule in the southern Irish Sea. -

Marine Ecology Progress Series 373:25–35 (2008)
The following appendices accompany the article Distributional overlap rather than habitat differentiation characterizes co-occurrence of bivalves in intertidal soft sediment systems Tanya J. Compton1, 2, 3,*, Tineke A. Troost1, Jaap van der Meer1, Casper Kraan1, 2, Pieter J. C. Honkoop1, Danny I. Rogers4, Grant B. Pearson3, Petra de Goeij1, Pierrick Bocher5, Marc S. S. Lavaleye1, Jutta Leyrer1, 2, Mick G. Yates6, Anne Dekinga1, Theunis Piersma1, 2 1Department of Marine Ecology, Royal Netherlands Institute for Sea Research (NIOZ), PO Box 59, 1790 AB Den Burg, Texel, The Netherlands 2Centre for Ecological and Evolutionary Studies, University of Groningen, PO Box 14, 9750 AA Haren, The Netherlands 3Western Australian Department of Environment and Conservation (DEC), WA Wildlife Research Centre, PO Box 51, Wanneroo, Western Australia 6065, Australia 4Institute of Land, Water and Society, Charles Sturt University, PO Box 789, Albury, New South Wales 2640, Australia 5Centre de Recherche sur les Ecosystèmes Littoraux Anthropisés (CRELA), UMR 6217, Pôle science, CNRS-IFREMER-Université de la Rochelle, La Rochelle 17042, France 6Centre for Ecology and Hydrology — Monks Wood, Abbots Ripton, Huntingdon, Cambridgeshire PE28 2LS, UK *Email: [email protected] Marine Ecology Progress Series 373:25–35 (2008) Appendix 1. Maps showing the gridding programme in each system. Benthic sampling points are shown as small dots; sediment sample points are indicated as larger dots. Median grain size values are shown in categories (Wentworth scale). Darker colours are muddy sample points, whereas lighter colours are sandier. The map of the German Wadden Sea has been divided to show the grid sampling at each location (A: 54° 32’ N, 8° 34’ E; B: 53° 59’ N, 8° 51’ E) 2 Appendix 1 (continued) Appendix 1 (continued) 3 4 Appendix 1 (continued) 5 Appendix 2. -

Macoma Balthica Class: Bivalvia; Heterodonta Order: Veneroida Family: Tellinidae
Phylum: Mollusca Macoma balthica Class: Bivalvia; Heterodonta Order: Veneroida Family: Tellinidae Taxonomy: Originally described as a height, and 11 mm in diameter (Oldroyd member of the genus Tellina, Macoma 1924). The illustrated specimen (from Coos balthica was the name of the Atlantic species. Bay) is 17.5 mm long. Our west coast clam was originally called M. Color: Distinct color is reddish, pale rose or inconspicua (Broderip and Sowerby 1829), white and is sometimes bluish or yellow but they are now generally considered to be (Oldroyd 1924; see Plate 17, Kozloff 1993). the same species (e.g., Vassallo, 1969, 1971; Coos Bay specimens are usually pink inside Haderlie and Abbott 1980). An extensive and out, but individuals from British Columbia, taxonomic history has yielded many Canada can have pink or yellow interiors synonyms for M. balthica. Some ambiguity (Quayle 1970). exists whether individuals from the General Morphology: Bivalve mollusks are southernmost reaches of the distribution on bilaterally symmetrical with two lateral valves east and west sides of the Atlantic should be or shells that are hinged dorsally and considered the same species (Beukema and surround a mantle, head, foot and viscera Meehan 1985) and some researchers (e.g., (see Plate 393B, Coan and Valentich-Scott Meehan 1985; Kamermans et al. 1990; 2007). Among the bivalves, the Heterodonta Luttikhuizen et al. 2012; Sanier et al. 2015) are characterized by ctenidia (or gills) that consider these allopatric populations to be are eulamellibranchiate, fused mantle subspecies (eastern Atlantic Macoma balthica margins and the presence of long siphons. balthica and western Atlantic Macoma Veneroid bivalves have well-developed hinge balthica rubra) that have been reproductively teeth and members of the family Tellinidae isolated for 2–3.5 million years (Luttikhuizen have short lateral hinge teeth (when present – et al.