Olfactory Stimulus
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Guide to Sensory Processing.Pdf
Guide to Sensory Processing Prepared by Allison Travnik, MSOTS Level II Fieldwork Student Project Kavitha N Krishnan MS OTR/L Fieldwork Instructor Sensory Processing In order to understand what is going on around us, we need to organize all of the incoming sensory information (Ayres, 2005). The sensory information involves what we see, smell, taste, hear, feel on our body, where our body is in relation to others, and how well we are balanced. This is a lot of information that our brains need to process in order to engage in productive behavior, learn, and form accurate perceptions. Proprioceptive Where are body is in space Tactile Auditory What we feel The noise on our skin around us Sensory Smell Processing The Sight difference What we see scents around us around us Oral Sensory Processing Vestibular The sensations Jean Ayres developed the sensory Our sense of Disorder + balance that food give integration (SI) theory. SI gives us in our mouth meaning to what our senses are recognizing. When the sensations are not being organized properly may notice some of the same qualities in the brain, Ayres compared it to about yourself.It is important to a traffic jam. The traffic jam of remember that everyone has some sensory information can lead to quirks about their sensory processing learning difficulties and problem whether it be a sensitivity to loud behavior (Ayres, 2005). Children noises or dislike of light touch. with Sensory Processing Disorder However the identification of SPD is (SPD) are struggling with this reserved for individuals whose traffic jam. sensory quirks are outside of the Sensory processing is a typical range and affect their daily dynamic and complex theory. -

Understanding Sensory Processing: Looking at Children's Behavior Through the Lens of Sensory Processing
Understanding Sensory Processing: Looking at Children’s Behavior Through the Lens of Sensory Processing Communities of Practice in Autism September 24, 2009 Charlottesville, VA Dianne Koontz Lowman, Ed.D. Early Childhood Coordinator Region 5 T/TAC James Madison University MSC 9002 Harrisonburg, VA 22807 [email protected] ______________________________________________________________________________ Dianne Koontz Lowman/[email protected]/2008 Page 1 Looking at Children’s Behavior Through the Lens of Sensory Processing Do you know a child like this? Travis is constantly moving, pushing, or chewing on things. The collar of his shirt and coat are always wet from chewing. When talking to people, he tends to push up against you. Or do you know another child? Sierra does not like to be hugged or kissed by anyone. She gets upset with other children bump up against her. She doesn’t like socks with a heel or toe seam or any tags on clothes. Why is Travis always chewing? Why doesn’t Sierra liked to be touched? Why do children react differently to things around them? These children have different ways of reacting to the things around them, to sensations. Over the years, different terms (such as sensory integration) have been used to describe how children deal with the information they receive through their senses. Currently, the term being used to describe children who have difficulty dealing with input from their senses is sensory processing disorder. _____________________________________________________________________ Sensory Processing Disorder -

Taste and Smell Disorders in Clinical Neurology
TASTE AND SMELL DISORDERS IN CLINICAL NEUROLOGY OUTLINE A. Anatomy and Physiology of the Taste and Smell System B. Quantifying Chemosensory Disturbances C. Common Neurological and Medical Disorders causing Primary Smell Impairment with Secondary Loss of Food Flavors a. Post Traumatic Anosmia b. Medications (prescribed & over the counter) c. Alcohol Abuse d. Neurodegenerative Disorders e. Multiple Sclerosis f. Migraine g. Chronic Medical Disorders (liver and kidney disease, thyroid deficiency, Diabetes). D. Common Neurological and Medical Disorders Causing a Primary Taste disorder with usually Normal Olfactory Function. a. Medications (prescribed and over the counter), b. Toxins (smoking and Radiation Treatments) c. Chronic medical Disorders ( Liver and Kidney Disease, Hypothyroidism, GERD, Diabetes,) d. Neurological Disorders( Bell’s Palsy, Stroke, MS,) e. Intubation during an emergency or for general anesthesia. E. Abnormal Smells and Tastes (Dysosmia and Dysgeusia): Diagnosis and Treatment F. Morbidity of Smell and Taste Impairment. G. Treatment of Smell and Taste Impairment (Education, Counseling ,Changes in Food Preparation) H. Role of Smell Testing in the Diagnosis of Neurodegenerative Disorders 1 BACKGROUND Disorders of taste and smell play a very important role in many neurological conditions such as; head trauma, facial and trigeminal nerve impairment, and many neurodegenerative disorders such as Alzheimer’s, Parkinson Disorders, Lewy Body Disease and Frontal Temporal Dementia. Impaired smell and taste impairs quality of life such as loss of food enjoyment, weight loss or weight gain, decreased appetite and safety concerns such as inability to smell smoke, gas, spoiled food and one’s body odor. Dysosmia and Dysgeusia are very unpleasant disorders that often accompany smell and taste impairments. -

Auditory System & Hearing
Auditory System & Hearing Chapters 9 part II Lecture 17 Jonathan Pillow Sensation & Perception (PSY 345 / NEU 325) Fall 2017 1 Cochlea: physical device tuned to frequency! • place code: tuning of different parts of the cochlea to different frequencies 2 The auditory nerve (AN): fibers stimulated by inner hair cells • Frequency selectivity: Clearest when sounds are very faint 3 Threshold tuning curves for 6 neurons (threshold = lowest intensity that will give rise to a response) Characteristic frequency - frequency to which the neuron is most sensitive threshold(dB) frequency (kHz) 4 Information flow in the auditory pathway • Cochlear nucleus: first brain stem nucleus at which afferent auditory nerve fibers synapse • Superior olive: brainstem region thalamus MGN in the auditory pathway where inputs from both ears converge • Inferior colliculus: midbrain nucleus in the auditory pathway • Medial geniculate nucleus (MGN): part of the thalamus that relays auditory signals to the cortex 5 • Primary auditory cortex (A1): First cortical area for processing audition (in temporal lobe) • Belt & Parabelt areas: areas beyond A1, where neurons respond to more complex characteristics of sounds 6 Basic Structure of the Mammalian Auditory System Comparing overall structure of auditory and visual systems: • Auditory system: Large proportion of processing before A1 • Visual system: Large proportion of processing after V1 7 Basic Structure of the Mammalian Auditory System Tonotopic organization: neurons organized spatially in order of preferred frequency • -

Review Article Olfactory Reference Syndrome
British Journal of Pharmaceutical and Medical Research Vol.04, Issue 01, Pg.1617-1625, January-February 2019 Available Online at http://www.bjpmr.org BRITISH JOURNAL OF PHARMACEUTICAL AND MEDICAL RESEARCH Review ISSN:2456-9836 ICV: 60.37 Article Murat Eren Özen, Murat Aydin Olfactory Reference Syndrome: A Separate Disorder Or Part Of A Spectrum 1Psychiatrist, Department of Psychiatry, Private Adana Hospital, Adana Büyük şehir Belediyesi kar şısı, No:23, Seyhan-Adana- Türkiye. 2Private Dental Clinics, Gazipa şa bulv. Emre apt n:6 (kitapsan kar şısı) k:2 d:5 Adana- Türkiye. http://drmurataydin.com ARTICLE INFO ABSTRACT This article provides a narrative review of the literature on olfactory reference syndrome Article History: Received on (ORS) to address issues focusing on its clinical features. Similarities and/or differences with other psychiatric disorders such as obsessive-compulsive spectrum disorders, social anxiety 10 th Jan, 2019 Peer Reviewed disorder (including a cultural syndrome; taijin kyofusho), somatoform disorders and hypochondriasis, delusional disorder are discussed. ORS is related to a symptom of taijin on 24 th Jan, 2019 Revised kyofusho (e.g. jikoshu-kyofu variant of taijin kyofusho) Although recognition of this syndromes more than a century provide consistent descriptions of its clinical features, the on 17 th Feb, 2019 Published limited data on this topic make it difficult to form a specific diagnostic criteria. The core on 24 th Feb, symptom of the patients with ORS is preoccupation with the belief that one emits a foul or 2019 offensive body odor, which is not perceived by others. Studies on ORS reveal some limitations. -

Neural Tracking of the Musical Beat Is Enhanced by Low-Frequency Sounds
Neural tracking of the musical beat is enhanced by low-frequency sounds Tomas Lenca, Peter E. Kellera, Manuel Varleta, and Sylvie Nozaradana,b,c,1 aMARCS Institute for Brain, Behaviour, and Development, Western Sydney University, Penrith, NSW 2751, Australia; bInstitute of Neuroscience (IONS), Université Catholique de Louvain, 1200 Woluwe-Saint-Lambert, Belgium; and cInternational Laboratory for Brain, Music, and Sound Research (BRAMS), Département de Psychologie, Faculté des Arts et des Sciences, Université de Montréal, Montréal, QC H3C 3J7, Canada Edited by Dale Purves, Duke University, Durham, NC, and approved June 28, 2018 (received for review January 24, 2018) Music makes us move, and using bass instruments to build the content (8, 9). Bass sounds are also crucial in music that en- rhythmic foundations of music is especially effective at inducing courages listeners to move (10, 11). people to dance to periodic pulse-like beats. Here, we show that There has been a recent debate as to whether evolutionarily this culturally widespread practice may exploit a neurophysiolog- shaped properties of the auditory system lead to superior tem- ical mechanism whereby low-frequency sounds shape the neural poral encoding for bass sounds (12, 13). One study using elec- representations of rhythmic input by boosting selective locking to troencephalography (EEG) recorded brain responses elicited by the beat. Cortical activity was captured using electroencephalog- misaligned tone onsets in an isochronous sequence of simulta- raphy (EEG) while participants listened to a regular rhythm or to a neous low- and high-pitched tones (12). Greater sensitivity to the relatively complex syncopated rhythm conveyed either by low temporal misalignment of low tones was observed when they tones (130 Hz) or high tones (1236.8 Hz). -
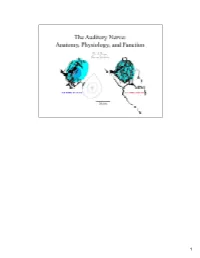
Auditory Nerve.Pdf
1 Sound waves from the auditory environment all combine in the ear canal to form a complex waveform. This waveform is deconstructed by the cochlea with respect to time, loudness, and frequency and neural signals representing these features are carried into the brain by the auditory nerve. It is thought that features of the sounds are processed centrally along parallel and hierarchical pathways where eventually percepts of the sounds are organized. 2 In mammals, the neural representation of acoustic information enters the brain by way of the auditory nerve. The auditory nerve terminates in the cochlear nucleus, and the cochlear nucleus in turn gives rise to multiple output projections that form separate but parallel limbs of the ascending auditory pathways. How the brain normally processes acoustic information will be heavily dependent upon the organization of auditory nerve input to the cochlear nucleus and on the nature of the different neural circuits that are established at this early stage. 3 This histology slide of a cat cochlea (right) illustrates the sensory receptors, the auditory nerve, and its target the cochlear nucleus. The orientation of the cut is illustrated by the pink line in the drawing of the cat head (left). We learned about the relationship between these structures by inserting a dye-filled micropipette into the auditory nerve and making small injections of the dye. After histological processing, stained single fibers were reconstruct back to their origin, and traced centrally to determine how they terminated in the brain. We will review the components of the nerve with respect to composition, innervation of the receptors, cell body morphology, myelination, and central terminations. -

What Is Sensory Integration?
WHAT IS SENSORY INTEGRATION? Sensory integration is the brain's innate ability to take in (register and perceive) information from the environment through the various senses, process and organize the information and then use it effectively. WHAT IS SENSORY INTEGRATION DYSFUNCTION? Sensory integration dysfunction is when the brain is unable to process the information received from the senses. When this occurs, the brain cannot analyze, organize or integrate this information to produce a functional outcome. POSSIBLE SYMPTOMS OF SENSORY INTEGRATION DYSFUNCTION Hyper or hypo sensitive to touch, movement, sight, sounds or smells. Activity level which is too high or too low. Coordination problems. Delays in speech, language, motor skills and academic achievement. Poor organization of behavior Poor self-concept (body awareness) THE VESTIBULAR SYSTEM Vestibular processing refers to the information that is provided by the receptors within the inner ear. These receptors are stimulated by movement of the head and input from other senses. This input tells where we are in relation to gravity, whether we are still or moving, how fast we are going, and in which direction. It also influences the development of balance, equilibrium, postural control and muscle tone. Vestibular input also plays an important role in helping to maintain a calm, alert state and keeping the level of arousal in the central nervous system balanced. An under-reactive vestibular system can contribute to distractibility and hyperactivity due to the lack of its modulating influence. Depending upon the situation, vestibular stimulation can either calm or stimulate and facilitate a more organized activity level. Children can be hyper and/or hypo-responsive to this type of input. -

36 | Sensory Systems 1109 36 | SENSORY SYSTEMS
Chapter 36 | Sensory Systems 1109 36 | SENSORY SYSTEMS Figure 36.1 This shark uses its senses of sight, vibration (lateral-line system), and smell to hunt, but it also relies on its ability to sense the electric fields of prey, a sense not present in most land animals. (credit: modification of work by Hermanus Backpackers Hostel, South Africa) Chapter Outline 36.1: Sensory Processes 36.2: Somatosensation 36.3: Taste and Smell 36.4: Hearing and Vestibular Sensation 36.5: Vision Introduction In more advanced animals, the senses are constantly at work, making the animal aware of stimuli—such as light, or sound, or the presence of a chemical substance in the external environment—and monitoring information about the organism’s internal environment. All bilaterally symmetric animals have a sensory system, and the development of any species’ sensory system has been driven by natural selection; thus, sensory systems differ among species according to the demands of their environments. The shark, unlike most fish predators, is electrosensitive—that is, sensitive to electrical fields produced by other animals in its environment. While it is helpful to this underwater predator, electrosensitivity is a sense not found in most land animals. 36.1 | Sensory Processes By the end of this section, you will be able to do the following: • Identify the general and special senses in humans • Describe three important steps in sensory perception • Explain the concept of just-noticeable difference in sensory perception Senses provide information about the body and its environment. Humans have five special senses: olfaction (smell), gustation (taste), equilibrium (balance and body position), vision, and hearing. -

Respiration, Neural Oscillations, and the Free Energy Principle
fnins-15-647579 June 23, 2021 Time: 17:40 # 1 REVIEW published: 29 June 2021 doi: 10.3389/fnins.2021.647579 Keeping the Breath in Mind: Respiration, Neural Oscillations, and the Free Energy Principle Asena Boyadzhieva1*† and Ezgi Kayhan2,3† 1 Department of Philosophy, University of Vienna, Vienna, Austria, 2 Department of Developmental Psychology, University of Potsdam, Potsdam, Germany, 3 Max Planck Institute for Human Cognitive and Brain Sciences, Leipzig, Germany Scientific interest in the brain and body interactions has been surging in recent years. One fundamental yet underexplored aspect of brain and body interactions is the link between the respiratory and the nervous systems. In this article, we give an overview of the emerging literature on how respiration modulates neural, cognitive and emotional processes. Moreover, we present a perspective linking respiration to the free-energy principle. We frame volitional modulation of the breath as an active inference mechanism Edited by: in which sensory evidence is recontextualized to alter interoceptive models. We further Yoko Nagai, propose that respiration-entrained gamma oscillations may reflect the propagation of Brighton and Sussex Medical School, United Kingdom prediction errors from the sensory level up to cortical regions in order to alter higher level Reviewed by: predictions. Accordingly, controlled breathing emerges as an easily accessible tool for Rishi R. Dhingra, emotional, cognitive, and physiological regulation. University of Melbourne, Australia Georgios D. Mitsis, Keywords: interoception, respiration-entrained neural oscillations, controlled breathing, free-energy principle, McGill University, Canada self-regulation *Correspondence: Asena Boyadzhieva [email protected] INTRODUCTION † These authors have contributed “The “I think” which Kant said must be able to accompany all my objects, is the “I breathe” which equally to this work actually does accompany them. -

Sensory Overamplification in Layer 5 Auditory Corticofugal Projection Neurons Following Cochlear Nerve Synaptic Damage
Corrected: Publisher correction ARTICLE DOI: 10.1038/s41467-018-04852-y OPEN Sensory overamplification in layer 5 auditory corticofugal projection neurons following cochlear nerve synaptic damage Meenakshi M. Asokan1,2, Ross S. Williamson1,3, Kenneth E. Hancock1,3 & Daniel B. Polley1,2,3 Layer 5 (L5) cortical projection neurons innervate far-ranging brain areas to coordinate integrative sensory processing and adaptive behaviors. Here, we characterize a plasticity in 1234567890():,; L5 auditory cortex (ACtx) neurons that innervate the inferior colliculus (IC), thalamus, lateral amygdala and striatum. We track daily changes in sound processing using chronic widefield calcium imaging of L5 axon terminals on the dorsal cap of the IC in awake, adult mice. Sound level growth functions at the level of the auditory nerve and corticocollicular axon terminals are both strongly depressed hours after noise-induced damage of cochlear afferent synapses. Corticocollicular response gain rebounded above baseline levels by the following day and remained elevated for several weeks despite a persistent reduction in auditory nerve input. Sustained potentiation of excitatory ACtx projection neurons that innervate multiple limbic and subcortical auditory centers may underlie hyperexcitability and aberrant functional coupling of distributed brain networks in tinnitus and hyperacusis. 1 Eaton-Peabody Laboratories, Massachusetts Eye and Ear Infirmary, Boston, MA 02114, USA. 2 Division of Medical Sciences, Harvard University, Boston, MA 02114, USA. 3 Department of Otolaryngology, Harvard Medical School, Boston, MA 02114, USA. Correspondence and requests for materials should be addressed to M.M.A. (email: [email protected]) NATURE COMMUNICATIONS | (2018) 9:2468 | DOI: 10.1038/s41467-018-04852-y | www.nature.com/naturecommunications 1 ARTICLE NATURE COMMUNICATIONS | DOI: 10.1038/s41467-018-04852-y he auditory system employs a variety of gain control to the IC and striatum33 or both to the IC and brainstem34). -

Review Inhibitory Neurotransmission, Plasticity and Aging in the Mammalian Central Auditory System
1781 The Journal of Experimental Biology 211, 1781-1791 Published by The Company of Biologists 2008 doi:10.1242/jeb.013581 Review Inhibitory neurotransmission, plasticity and aging in the mammalian central auditory system Donald M. Caspary1,*, Lynne Ling1, Jeremy G. Turner1,2 and Larry F. Hughes1 1Southern Illinois University School of Medicine, Springfield, IL 62794, USA and 2Illinois College, Jacksonville, IL 62650, USA *Author for correspondence (e-mail: [email protected]) Accepted 4 February 2008 Summary Aging and acoustic trauma may result in partial peripheral deafferentation in the central auditory pathway of the mammalian brain. In accord with homeostatic plasticity, loss of sensory input results in a change in pre- and postsynaptic GABAergic and glycinergic inhibitory neurotransmission. As seen in development, age-related changes may be activity dependent. Age-related presynaptic changes in the cochlear nucleus include reduced glycine levels, while in the auditory midbrain and cortex, GABA synthesis and release are altered. Presumably, in response to age-related decreases in presynaptic release of inhibitory neurotransmitters, there are age-related postsynaptic subunit changes in the composition of the glycine (GlyR) and GABAA (GABAAR) receptors. Age-related changes in the subunit makeup of inhibitory pentameric receptor constructs result in altered pharmacological and physiological responses consistent with a net down-regulation of functional inhibition. Age-related functional changes associated with glycine neurotransmission in dorsal cochlear nucleus (DCN) include altered intensity and temporal coding by DCN projection neurons. Loss of synaptic inhibition in the superior olivary complex (SOC) and the inferior colliculus (IC) likely affect the ability of aged animals to localize sounds in their natural environment.