Ate Complexes Frorn Diastereomeric Anci Enantiomeric Alkylmercury
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Enlarging Knowledge on Lager Beer Volatile Metabolites Using Multidimensional Gas Chromatography
foods Article Enlarging Knowledge on Lager Beer Volatile Metabolites Using Multidimensional Gas Chromatography Cátia Martins 1 , Tiago Brandão 2, Adelaide Almeida 3 and Sílvia M. Rocha 1,* 1 Departamento de Química & LAQV-REQUIMTE, Universidade de Aveiro, Campus Universitário Santiago, 3810-193 Aveiro, Portugal; [email protected] 2 Super Bock Group, Rua do Mosteiro, 4465-703 Leça do Balio, Portugal; [email protected] 3 Departamento de Biologia & CESAM, Universidade de Aveiro, Campus Universitário Santiago, 3810-193 Aveiro, Portugal; [email protected] * Correspondence: [email protected]; Tel.: +351-234-401-524 Received: 30 July 2020; Accepted: 6 September 2020; Published: 11 September 2020 Abstract: Foodomics, emergent field of metabolomics, has been applied to study food system processes, and it may be useful to understand sensorial food properties, among others, through foods metabolites profiling. Thus, as beer volatile components represent the major contributors for beer overall and peculiar aroma properties, this work intends to perform an in-depth profiling of lager beer volatile metabolites and to generate new data that may contribute for molecules’ identification, by using multidimensional gas chromatography. A set of lager beers were used as case-study, and 329 volatile metabolites were determined, distributed over 8 chemical families: acids, alcohols, esters, monoterpenic compounds, norisoprenoids, sesquiterpenic compounds, sulfur compounds, and volatile phenols. From these, 96 compounds are reported for the first time in the lager beer volatile composition. Around half of them were common to all beers under study. Clustering analysis allowed a beer typing according to production system: macro- and microbrewer beers. Monoterpenic and sesquiterpenic compounds were the chemical families that showed wide range of chemical structures, which may contribute for the samples’ peculiar aroma characteristics. -

Specific Determination of Airborne Sulfates and Sulfuric Acid
Louisiana State University LSU Digital Commons LSU Historical Dissertations and Theses Graduate School 1977 Specific etD ermination of Airborne Sulfates and Sulfuric Acid. Purnendu Kumar Dasgupta Louisiana State University and Agricultural & Mechanical College Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_disstheses Recommended Citation Dasgupta, Purnendu Kumar, "Specific eD termination of Airborne Sulfates and Sulfuric Acid." (1977). LSU Historical Dissertations and Theses. 3152. https://digitalcommons.lsu.edu/gradschool_disstheses/3152 This Dissertation is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Historical Dissertations and Theses by an authorized administrator of LSU Digital Commons. For more information, please contact [email protected]. INFORMATION TO USERS This material was produced from a microfilm copy of the original document. While the most advanced technological means to photograph and reproduce this document have been used, the quality is heavily dependent upon the quality of the original submitted. The following explanation of techniques is provided to help you understand markings or patterns which may appear on this reproduction. 1. The sign or "target" for pages apparently lacking from the document photographed is "Missing Page(s)". If it was possible to obtain the missing page(s) or section, they are spliced into the film along with adjacent pages. This may have necessitated cutting thru an image and duplicating adjacent pages to insure you complete continuity. 2. When an image on the film is obliterated with a large round black mark, it is an indication that the photographer suspected that the copy may have moved during exposure and thus cause a blurred image. -
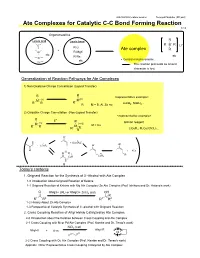
Ate Complexes for Catalytic C-C Bond Forming Reaction 1/13
2007/09/08 Literature seminer Tomoyuki Mashiko (M1 part) Ate Complexes for Catalytic C-C Bond Forming Reaction 1/13 Organometallics R Lewis acid Lewis base - B R B R Li+ R-Li + Ate complex R Al R-MgX etc R-Na etc Zn etc • Central metal is anionic. Cu The reaction proceeds as anionic character is lost. Generalization of Reaction Pathways for Ate Complexes 1) Non-Oxidative Charge Cancellation (Ligand Transfer) R R <representative example> M- (n) M(n) R R R LiAlH , NaBH .. R- M = B, Al, Zn etc 4 4. 2) Oxidative Charge Cancellation (Non-Ligand Transfer) <representative example> R + E R Gilman reagent M- (n) (n+2) R R M M = Cu R E LiCuR , R Cu(CN)Li .. R 2 2 2. O- Ⅱ + + [LiCuR2] - Ⅰ - O + LiCuR2 O Ⅰ O Li + RCu O R Ⅲ Ⅰ R CuR CuR2 0 Today's contents 1. Grignard Reaction for the Synthesis of 3°-Alcohol with Ate Complex 1-0 Introduction about Grignard Reaction of Ketone 1-1 Grignard Reaction of Ketone with Mg Ate Complex/ Zn Ate Complex (Prof. Ishihara and Dr. Hatano's work) O RMgX+ 2RLi or RMgCl+ ZnCl2 (cat) OH R R1 R2 R1 R2 1-2 History About Zn Ate Complex 1-3 Perspective of Catalytic Synthesis of 3°-alcohol with Grignard Reaction 2. Cross Coupling Reaction of Alkyl Halide Catalyzed by Ate Complex 2-0 Introduction about the Relation between Cross Coupling and Ate Complex 2-1 Cross Coupling with Ni or Pd Ate Complex (Prof. Kambe and Dr. Terao's work) NiCl2 (cat) Alkyl-R Alkyl-X + Ⅱ R-m Ni 2-2 Cross Coupling with Cu Ate Complex (Prof. -

Clark, Hobart, and Neu 1995
Waste Isolation Pilot Plant Compliance Certification Application Reference 135 Clark, D.L., D.E. Hobart, and M.P. Neu. 1995. Actinide Carbonate Complexes and Their Importance in Actinide Environmental Chemistry, Chem Revs. Vol. 95; 25-48. Submitted in accordance with 40 CFR $194.13, Submission of Reference Materials. Carera, ;., Neurcan. 3.p.. - 986 "Est~rncaonoi ?x:ier Parcrneters L'nae; Panslent anc' S:eadu Sco:z ~oi~icions,2. Unlacaness, Stc;z.:ird, and solucion ftiqo:ithrns." 9. Clark, D.L.. Floba~.D.E., Neu, M.P.. 1995 '9ctinicz Carbonate Complexes ana Thslr Irnporcccca in Ect;nide Environmencai G,~mrsny."=?em Revs. ',Jot. 05, 25-48. ON ' ; x 28.C3 398.00 Cleveland, J.M.. i 9* nGit:calRevleu of Plutonium Eov~lbrioof Environrnencal Concern. In Moueirng in Equmus S;lstms: Smrct:on, So~ubrlrtuanu tlnq of t9e Emencan Chernrcc~Societu, Pdiarnr Beacn, Fi, Series: 3521 -336. Cti~- ; x CLO.CC 220.30 . - , I. 2av1s.G.B.. Jcnnsco i 984 'Ccxxenc on Cmcamincnc Tianspor: :n fracturad PONS Media: fcr s Sjscern cS ~rciielFrcc:vres" 5y SLG~C~U,C.A., and Fmd, E.O.,' Rasc~rcesRzsecrcn, \jot. 23,i\.'o. 9. s?. : 321 - 1 322, Szpt. ., -- ? 984. 1 Qtl; I i x i 3.:: ' 48.50 , , -. - 4 Actinide Carbonate Complexes and Their Importance in Actinide Environmental Chemistry I David L. Clark,'~~~David E. Hobart,lb and Mary P. Neda Chemical Science and Technology Division, Los Alamas National Laboratory, Los Alamos, Mw Me& 87545, l?eThe Sciems DMm, Lawrence Berkeley Laboratory, BeBerky, California 94720, and UE G. T. Seabog imWe for Transactinium Scienae, I Livemre, California 94551 I Received May 16, 1994 (Revised Manuscript ReceM September 16, 1994) Table 1. -

Volatile Organic Compounds from Books
1 Measuring the emission of volatile organic compounds from books Velson Horie Research Project Manager The British Library What is happening to our books? presevation of folding endurance DP X. Zou, T. Uesaka, N. Gurnagul, Prediction of paper permanence by accelerated aging. Part I: Kinetic analysis of the 3 aging process , Cellulose, 1996, 3, 243-267. A Few Statistics •Formal beginning in 1753 as the library of The British Museum •The British Library formed in 1973 from many collections •New St Pancras building opened in 1998 •150m collection items on 640km of shelves, •£131m budget, 1900 staff 4 Additional Storage Programme - Boston Spa •7 million collection items •263 km, 12,000 tonne of stock •Reduced oxygen (16%) •Robotic book handling •What are the long term effects? 5 Preserving Newspapers •33 km of stock •5,300 tonne of stock •1.4 tonne/y VOC production •3,800 years till all evaporated 6 Major UK libraries and archives Cambridge University Library (CUL) 7m printed items The British Library (BL) 150m items National Library of Scotland (NLS) 14m items National Library of Wales (NLW) 6m printed items Oxford University Library(OULS) 11m items Trinity College Dublin Library (TCD) 4m printed items The National Archives (TNA) National Archives of Scotland (NAS) 7 Condition assessment Preservation Assessment Survey Strength Colour pH Molecular weight Furnish SurveNIR VOCs 8 The “real thing” is important to people E-books sales have been slow to take off. CafeScribe is sending every e-textbook purchaser a scratch and sniff sticker with a musty “old book” smell. By placing these stickers on their computers, they can give their e-books the same musty book smell they know and love from used textbooks. -

Development of Novel Methodologies in Organic Synthesis Based on Ate Complex Formation
No.171 No.171 ResearchResearch ArticleArticle Development of Novel Methodologies in Organic Synthesis Based on Ate Complex Formation Keiichi Hirano1* and Masanobu Uchiyama1,2* 1 Graduate School of Pharmaceutical Sciences, The University of Tokyo,7-3-1 Hongo, Bunkyo-ku, Tokyo 113-0033, Japan 2 Elements Chemistry Laboratory, RIKEN,2-1 Hirosawa, Wako-shi, Saitama 351-0198, Japan E-mail: [email protected]; [email protected] Abstract: We have been focusing on development of novel synthetic methodologies based on ate complex formation. By fine-tuning of coordination environments of various main-group metals (Zn, Al, and B) as well as transition metal (Cu), a wide range of carbon-carbon bond and carbon-heteroatom bond (C–I, C–O, C–N, C–H, C–Si, and C–B) formation reactions have been realized in highly regio- and chemoselective manner taking advantage of characteristics of the elements. Keywords: Ate complex, Halogen-metal exchange, Deprotonative metalation, Chemoselectivity, Regioselectivity 1. Introduction 2. Ate Complexes Ate complexes are known to be anionic organometallic Discovery of the first mono-anion type zincate, Na[ZnEt3], complexes, whose central metals have increased valence by by James A. Wanklyn dates back to 1859,1) and even di-anion accepting Lewis basic ligands to their vacant orbitals. They type zincate, Li2[ZnMe4], was already reported by Dallas T. are attractive chemical species offering tunable reactivity due Hurd in 1948.2) Zincates experienced a long blank after these to their flexible choices of central metals, counter cations, important findings before they started to attract attentions and coordination environments. -

Effect of Substituents of Cerium Pyrazolates and Pyrrolates on Carbon Dioxide Activation
molecules Article Effect of Substituents of Cerium Pyrazolates and Pyrrolates on Carbon Dioxide Activation Uwe Bayer, Adrian Jenner, Jonas Riedmaier, Cäcilia Maichle-Mössmer and Reiner Anwander * Institute of Inorganic Chemistry, Eberhard Karls Universität Tübingen, Auf der Morgenstelle 18, 72076 Tübingen, Germany; [email protected] (U.B.); [email protected] (A.J.); [email protected] (J.R.); [email protected] (C.M.-M.) * Correspondence: [email protected] Abstract: Homoleptic ceric pyrazolates (pz) Ce(RR’pz)4 (R = R’ = tBu; R = R’ = Ph; R = tBu, R’ = Me) were synthesized by the protonolysis reaction of Ce[N(SiHMe2)2]4 with the corresponding pyrazole derivative. The resulting complexes were investigated in their reactivity toward CO2, revealing a significant influence of the bulkiness of the substituents on the pyrazolato ligands. The efficiency of the CO2 insertion was found to increase in the order of tBu2pz < Ph2pz < tBuMepz < Me2pz. For comparison, the pyrrole-based ate complexes [Ce2(pyr)6(m-pyr)2(thf)2][Li(thf)4]2 (pyr = pyrro- lato) and [Ce(cbz)4(thf)2][Li(thf)4] (cbz = carbazolato) were obtained via protonolysis of the cerous ate complex Ce[N(SiHMe2)2]4Li(thf) with pyrrole and carbazole, respectively. Treatment of the pyrrolate/carbazolate complexes with CO2 seemed promising, but any reversibility could not be observed. Keywords: cerium; pyrazoles; pyrroles; carbazoles; carbon dioxide Citation: Bayer, U.; Jenner, A.; Riedmaier, J.; Maichle-Mössmer, C.; Anwander, R. Effect of Substituents of 1. Introduction Cerium Pyrazolates and Pyrrolates on Rare-earth–metal complexes are capable of efficiently activating carbonylic com- Carbon Dioxide Activation. -

(12) Patent Application Publication (10) Pub. No.: US 2011/0028732 A1 Trauth Et Al
US 2011 0028732A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2011/0028732 A1 Trauth et al. (43) Pub. Date: Feb. 3, 2011 (54) NITRATED HYDROCARBONS, (86). PCT No.: PCT/US2009/0399.01 DERIVATIVES, AND PROCESSES FOR THEIR MANUFACTURE S371 (c)(1), (2), (4) Date: Sep. 27, 2010 (75) Inventors: Daniel M. Trauth, Crystal Lake, IL Related U.S. Application Data (US); George D. Green, Cary, IL (US); Raymond J. Swedo, Mt. (60) Eyal application No. 61/045.380, filed on Apr. Prospect, IL (US); Richard L. s James, Eros, LA (US); Ian A. Publication Classification Tomlinson, Midland, MI (US) (51) Int. Cl. C07D 263/04 (2006.01) Correspondence Address: C07C 205/05 (2006.01) The Dow Chemical Company C07C 205/01 (2006.01) P.O. BOX 1967, 2040 Dow Center CD7C 205/06 (2006.01) Midland, MI 48641 (US) C07C 215/02 (2006.01) C07C 239/08 (2006.01) (73) Assignees: ANGUS CHEMICAL (52) U.S. Cl. ......... 548/215; 7.3,So COMPANY, Buffalo Grove, IL s s (US): GLOBAL (57) ABSTRACT TECHNOLOGIES INC. , MidlandM1Clland, Provided is a process for the formation of nitrated compounds MI (US) by the nitration of hydrocarbon compounds with- dilute- - nitric acid. Also provided are processes for preparing industrially (21) Appl. No.: 12/934,817 useful downstream derivatives of the nitrated compounds, as well as novel nitrated compounds and derivatives, and meth (22) PCT Filed: Apr. 8, 2009 ods of using the derivatives in various applications. US 2011/0028732 A1 Feb. 3, 2011 NITRATED HYDROCARBONS, 0009. The invention further provides methods of using the DERIVATIVES, AND PROCESSES FOR THEIR nitrated hydrocarbons and derivatives thereof in various MANUFACTURE applications. -

Exposing the Hidden Complexity of Stoichiometric and Catalytic Metathesis Reactions by Elucidation of Mg-Zn Hybrids
Exposing the hidden complexity of stoichiometric and catalytic metathesis reactions by elucidation of Mg-Zn hybrids Eva Hevia1, Jonathan Z. Chua, Pablo García-Álvarez, Alan R. Kennedy, and Matthew D. McCall WestCHEM, Department of Pure and Applied Chemistry, University of Strathclyde, Glasgow, UK, G1 1XL Edited by Jack Halpern, University of Chicago, Chicago, IL, and approved January 14, 2010 (received for review November 17, 2009) Studying seemingly simple metathesis reactions between ZnCl2 role in these vital, new synthetic methodologies. In fact, the num- and t BuMgCl has, surprisingly, revealed a much more complex ber of organometallic compounds combining magnesium and chemistry involving mixed magnesium-zinc compounds that could zinc within the same molecule is scarce (7, 8), contrasting with be regarded as Mg-Zn hybrids. Thus, the reaction of equimolar the numerous reports on the synthesis of mixed-metal com- t amounts of ZnCl2 and BuMgCl reveals the formation of the unpre- pounds (ates) containing an alkali-metal with either magnesium μ t cedented mixed Mg-Zn complex [ðTHFÞ4Mgð -ClÞ2Znð BuÞðClÞ] (1), (magnesiates), or zinc (zincates), that show enhanced reactivity as a result of the co-complexation of the two anticipated exchange beyond the confines of the monometallic reagents from which products of the metathesis. This magnesium zincate adopts a con- they are derived (9–11). tacted ion-pair structure, closely related to Knochel’s pioneering In situ metathetical approaches are by far the most common “Turbo” Grignard reagents. Furthermore, a second coproduct iden- vehicles of choice to prepare either these mixed magnesium-zinc tified in this reaction is the solvent-separated mixed magnesium- putative intermediates or neutral organozinc reagents. -

Cambridge CB2 3EG (Received 14 June 1990)
Journal of Physiology (1991), 437, pp. 431-448 431 With 11 figures Printed in Great Britain ACTIONS OF n-ALCOHOLS ON NICOTINIC ACETYLCHOLINE RECEPTOR CHANNELS IN CULTURED RAT MYOTUBES BY R. D. MURRELL*, M. S. BRAUNt AND THE LATE D. A. HAYDON From the Physiological Laboratory, University of Cambridge, Downing Street, Cambridge CB2 3EG (Received 14 June 1990) SUMMARY 1. The actions of the n-alcohols from pentanol to dodecanol on nicotinic acetylcholine receptor (nAChR) channels were investigated by recording single ACh- activated channel activity from inside-out membrane patches isolated from cultured rat myotubes. Alcohols were applied to the cytoplasmic side of the membrane; aqueous concentrations ranged from 11 7 mM-pentanol to 0-02 mm-dodecanol. 2. The intermediate-chain alcohols (pentanol to octanol) caused channel currents to fluctuate between the fully open and closed state level so that openings occurred in bursts interrupted by brief gaps. Closed time distributions were fitted well with two exponential components, the fast component representing the closures within a burst. The number of gaps within a burst was dependent on alcohol concentration whereas gap duration was independent of concentration but increased with increasing chain length of the alcohol up to octanol. 3. Nonanol and decanol reduced the mean duration of bursts of openings but did not cause an increase in the number of short closed intervals within a burst. Beyond decanol there was a decline in the ability of the n-alcohols to affect channel function. A saturated solution of undecanol (0-07 mM) reduced the mean open time by 33+17 %, whereas a saturated solution of dodecanol had no significant effect. -

KUNDU-THESIS.Pdf
Acknowledgement I would like to thank my advisor Prof. Zachary T. Ball for teaching me everything I know in synthetic skills during my first year and guiding me through the rest of my time here at Rice. He has helped me better myself through constant constructive criticism, both in research and writing. I would also like to thank past and present Ball group members, Dr. Brian Popp, Dr. Alex Zaykov, Dr. Jessica Herron, Vincenzo Russo, Ramya Sambasivan, Cara Bovet, Zhen Chen, Dr. Jane Coughlin, Farrukh Vohidov, Matt Minus, Rob Ferguson, Julian Cooper. They have been a very good support in discussing research, exchanging ideas, and have become great pals in the last few years. Especially, Brian and Ramya’s encouragement in tough times is really valued. I thank my friends Rajkishore Barik and Meenu Adhikari for making Houston a second home. Again, Ramya Sambasivan for being a very good friend and always a willing ear to vent out frustrations associated with the graduate life and Avani Verma for bringing all kinds of fun in life and being so encouraging during my panic moments in thesis writing process. I would like to take this opportunity to express my gratitude and respect for my father Kuru Ram, and mother Amita Kundu, for the confidence and faith they always had in me, my sister Aruna Hajra who always supported me in my endeavors. I would like to thank my fiancée Soumya Sarkar, for his never wavering trust, love and support throughout my Ph.D in our 6 yr long, long- distance relation. Last but not least thanks to everyone who cared! -Rituparna Kundu Abstract Developing Dirhodium-Complexes for Protein Inhibition and Modification & Copper-Catalyzed Remote Chlorination of Alkyl-Hydroperoxides by Rituparna Kundu The work describes the development of a new class of protein-inhibitors for protein-protein interactions, based on metallopeptides comprised of a dirhodium metal center. -

Chewing Gum Containing High-Potency Sweetener Particles with Modified Zein Coating
Europaisches Patentamt 19 European Patent Office Office europeen des brevets © Publication number: 0 427 796 B1 12 EUROPEAN PATENT SPECIFICATION @ Date of publication of patent specification : © int. ci.5: A23G 3/30, A23L 1/236, 29.09.93 Bulletin 93/39 A23L 1/22 (2j) Application number : 90900603.3 (22) Date of filing : 17.11.89 © International application number : PCT/US89/05159 @ International publication number : WO 90/06062 14.06.90 Gazette 90/14 © CHEWING GUM CONTAINING HIGH-POTENCY SWEETENER PARTICLES WITH MODIFIED ZEIN COATING. © Priority: 02.12.88 US 279215 (56) References cited : WPI, FILE SUPPLIER, Derwent Publications @ Date of publication of application : Ltd., London, GB; & JP-B-45012759 22.05.91 Bulletin 91/21 © Proprietor : WM. WRIGLEY JR. COMPANY © Publication of the grant of the patent : 410 North Michigan Avenue 29.09.93 Bulletin 93/39 Chicago Illinois 60611 (US) @ Designated Contracting States : @ Inventor : COURTRIGHT, Steven, B. AT BE CH DE FR GB IT LI LU NL SE 1629 Brummel Evanston, IL 60202 (US) (56) References cited : Inventor : BARRETT, Kevin, F. EP-A- 0 067 595 7202 Western Avenue EP-A- 0 320 522 Darien, IL 60559 (US) WO-A-89/03170 US-A- 29 682 © Representative : Baverstock, Michael George US-A- 3 262 788 Douglas et al US-A- 3 753 739 BOULT, WADE & TENNANT 27 Furnival Street US-A- 3 922 354 London, EC4A 1PQ (GB) US-A- 3 928 633 US-A- 3 956 507 US-A- 3 962 468 US-A- 4 004 039 US-A- 4 049 706 US-A- 4 139 639 US-A- 4 230 687 US-A- 4 269 860 US-A- 4 384 004 US-A- 4 384 005 US-A- 4 495 213 US-A- 4 497 835 US-A- 4 517 214 US-A- 4 554 167 US-A- 4 556 565 CO US-A- 4 568 560 CO US-A- 4 579 747 o> US-A- 4 597 970 US-A- 4 634 593 h- CM Note : Within nine months from the publication of the mention of the grant of the European patent, any person may give notice to the European Patent Office of opposition to the European patent granted.