Neuroanatomy Research Advances
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Olfactory Sulcus Morphology in Teenagers with First-Presentation
Psychiatry Research: Neuroimaging 292 (2019) 1–4 Contents lists available at ScienceDirect Psychiatry Research: Neuroimaging journal homepage: www.elsevier.com/locate/psychresns Olfactory sulcus morphology in teenagers with first-presentation borderline personality disorder T ⁎ Tsutomu Takahashia, , Yumiko Nishikawaa, Dennis Velakoulisb, Michio Suzukia, Patrick D. McGorryc,d, Christos Pantelisb, Andrew M. Chanenc,d a Department of Neuropsychiatry, University of Toyama Graduate School of Medicine and Pharmaceutical Sciences, 2630 Sugitani, Toyama 930-0194, Japan b Melbourne Neuropsychiatry Centre, Department of Psychiatry, The University of Melbourne and Melbourne Health, Melbourne, Australia c Orygen, the National Centre of Excellence in Youth Mental Health, Melbourne, Australia d Centre for Youth Mental Health, The University of Melbourne, Melbourne, Australia ARTICLE INFO ABSTRACT Keywords: Gray matter reduction of the orbitofrontal cortex (OFC) has been reported in borderline personality disorder Neurodevelopment (BPD), but it remains unknown whether the BPD patients exhibit morphologic changes of the olfactory sulcus, a Orbitofrontal cortex potential marker of forebrain development located on the OFC. We used magnetic resonance imaging to in- Trauma vestigate the length and depth of the olfactory sulcus in 20 teenagers (15 females and 5 males) with first- Impulsivity presentation BPD and 20 healthy controls (15 females and 5 males). While there was no group difference in the Magnetic resonance imaging length of the sulcus, the BPD patients (especially those with a history of trauma) had a significantly shallower right olfactory sulcus compared with controls. In addition, sulcus depth was negatively correlated with the severity of impulsivity and affective instability in the BPD patients. These preliminary findings may suggest a significant role of environmental risk factors (i.e., trauma exposure) during childhood to adolescence in the neurobiology of BPD. -

Trafficking and Signaling of Parkin-Associated
Distribution Agreement In presenting this thesis or dissertation as a partial fulfillment of the requirements for an advanced degree from Emory University, I hereby grant to Emory University and its agents the non-exclusive license to archive, make accessible, and display my thesis or dissertation in whole or in part in all forms of media, now or hereafter known, including display on the world wide web. I understand that I may select some access restrictions as part of the online submission of this thesis or dissertation. I retain all ownership rights to the copyright of the thesis or dissertation. I also retain the right to use in future works (such as articles or books) all or part of this thesis or dissertation. Signature: _____________________________ ______________ Jill Harley Dunham Date Trafficking and Signaling of Parkin-Associated Endothelin-Like Receptor GPR37 By Jill Harley Dunham B.S. The University of Georgia, 2003 Graduate Division of Biological and Biomedical Sciences Program in Molecular and Systems Pharmacology ________________________________ Randy Hall, Ph.D. Adviser _____________________________ Allan Levey, M.D., Ph.D. Committee Member _____________________________ John Hepler, Ph.D. Committee Member _____________________________ Lian Li, Ph.D. Committee Member Accepted: ________________________________ Lisa A. Tedesco, Ph.D. Dean of the Graduate School ________________________________ Date TRAFFICKING AND SIGNALING OF THE PARKIN-ASSOCIATED ENDOTHELIN-LIKE RECEPTOR GPR37 By Jill Harley Dunham B.S., University of Georgia, -

Ficha Catalográfica
Naiani Ferreira Marques Guanosina previne alterações mitocondriais, o efeito tipo- depressivo e o déficit olfatório induzido por modelos experimentais da doença de Parkinson Tese submetida ao Programa de Pós-graduação em Bioquímica do Centro de Ciências Biológicas da Universidade Federal de Santa Catarina como requisito parcial à obtenção do grau de doutora em Bioquímica. Orientadora: Profa. Dra. Carla Inês Tasca Florianópolis - SC 2019 Ficha de identificação da obra elaborada pelo autor, através do Programa de Geração Automática da Biblioteca Universitária da UFSC. Marques, Naiani Ferreira Guanosina previne alterações mitocondriais, o efeito tipo-depressivo e o déficit olfatório induzido por modelos experimentais da doença de Parkinson / Naiani Ferreira Marques ; orientadora, Carla Inês Tasca, 2019. 216 p. Tese (doutorado) - Universidade Federal de Santa Catarina, Centro de Ciências Biológicas, Programa de Pós-Graduação em Bioquímica, Florianópolis, 2019. Inclui referências. 1. Bioquímica. 2. Doença de Parkinson. 3. Guanosina. 4. 6-OHDA. 5. MPTP. I. Tasca, Carla Inês. II. Universidade Federal de Santa Catarina. Programa de Pós-Graduação em Bioquímica. III. Título. Dedico esse trabalho aos meus pais Jairo e Cleusa que sempre trabalharam muito para garantir a minha educação e sempre foram meus maiores apoiadores. AGRADECIMENTOS Agradeço primeiramente aos meus pais Jairo e Cleusa por toda a base, pelos ensinamentos e pelo apoio incondicional em todas as fases da minha educação e da minha vida. A sabedoria de vocês sempre será meu exemplo em todos os aspectos. Agradeço muito a minha orientadora Profa Dra Carla Tasca pela experiência, pelo conhecimento, pelo seu tempo dedicado ao meu trabalho, pela paciência e por todas as oportunidades que estar em seu laboratório me proporcionaram. -

Contribution of Enterococcus Faecalis to Urinary Tract Infection
Western University Scholarship@Western Electronic Thesis and Dissertation Repository 3-29-2018 3:00 PM Contribution of Enterococcus faecalis to urinary tract infection Samantha Ann Whiteside The University of Western Ontario Supervisor Reid, Gregor The University of Western Ontario Co-Supervisor Burton, Jeremy P. The University of Western Ontario Graduate Program in Microbiology and Immunology A thesis submitted in partial fulfillment of the equirr ements for the degree in Doctor of Philosophy © Samantha Ann Whiteside 2018 Follow this and additional works at: https://ir.lib.uwo.ca/etd Part of the Bacterial Infections and Mycoses Commons Recommended Citation Whiteside, Samantha Ann, "Contribution of Enterococcus faecalis to urinary tract infection" (2018). Electronic Thesis and Dissertation Repository. 5270. https://ir.lib.uwo.ca/etd/5270 This Dissertation/Thesis is brought to you for free and open access by Scholarship@Western. It has been accepted for inclusion in Electronic Thesis and Dissertation Repository by an authorized administrator of Scholarship@Western. For more information, please contact [email protected]. Abstract The purpose of this thesis was to increase understanding of enterococcal urinary tract infection (UTI), in particular, the response of Enterococcus to antibiotic prophylaxis in vitro and in vivo and enterococcal communication with the bladder. We studied the in vitro effects of trimethoprim-sulfamethoxazole (TMP/SMX) and nitrofurantoin, two of the most commonly used antibiotic treatments for the management of both UTI and recurrent UTI (RUTI), on Enterococcus faecalis attachment to urothelial cells. In doing so, we documented increases in bacterial attachment at growth inhibitory concentrations of nitrofurantoin, but not TMP/SMX. This increased virulence did not correlate with increased expression of virulence factors but was correlated with increased expression of three putative genes. -
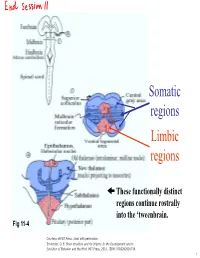
Lecture 12 Notes
Somatic regions Limbic regions These functionally distinct regions continue rostrally into the ‘tweenbrain. Fig 11-4 Courtesy of MIT Press. Used with permission. Schneider, G. E. Brain structure and its Origins: In the Development and in Evolution of Behavior and the Mind. MIT Press, 2014. ISBN: 9780262026734. 1 Chapter 11, questions about the somatic regions: 4) There are motor neurons located in the midbrain. What movements do those motor neurons control? (These direct outputs of the midbrain are not a subject of much discussion in the chapter.) 5) At the base of the midbrain (ventral side) one finds a fiber bundle that shows great differences in relative size in different species. Give examples. What are the fibers called and where do they originate? 8) A decussating group of axons called the brachium conjunctivum also varies greatly in size in different species. It is largest in species with the largest neocortex but does not come from the neocortex. From which structure does it come? Where does it terminate? (Try to guess before you look it up.) 2 Motor neurons of the midbrain that control somatic muscles: the oculomotor nuclei of cranial nerves III and IV. At this level, the oculomotor nucleus of nerve III is present. Fibers from retina to Superior Colliculus Brachium of Inferior Colliculus (auditory pathway to thalamus, also to SC) Oculomotor nucleus Spinothalamic tract (somatosensory; some fibers terminate in SC) Medial lemniscus Cerebral peduncle: contains Red corticospinal + corticopontine fibers, + cortex to hindbrain fibers nucleus (n. ruber) Tectospinal tract Rubrospinal tract Courtesy of MIT Press. Used with permission. Schneider, G. -

The Multi-Dimensional Contributions of Prefrontal Circuits to Emotion Regulation During Adulthood and Critical Stages of Development
brain sciences The Multi-Dimensional Contributions of Prefrontal Circuits to Emotion Regulation during Adulthood and Critical Stages of Development Edited by Angela Roberts Printed Edition of the Special Issue Published in Brain Sciences www.mdpi.com/journal/brainsci The Multi-Dimensional Contributions of Prefrontal Circuits to Emotion Regulation during Adulthood and Critical Stages of Development The Multi-Dimensional Contributions of Prefrontal Circuits to Emotion Regulation during Adulthood and Critical Stages of Development Special Issue Editor Angela Roberts MDPI • Basel • Beijing • Wuhan • Barcelona • Belgrade Special Issue Editor Angela Roberts University of Cambridge UK Editorial Office MDPI St. Alban-Anlage 66 4052 Basel, Switzerland This is a reprint of articles from the Special Issue published online in the open access journal Actuators (ISSN 2076-0825) from 2018 to 2019 (available at: https://www.mdpi.com/journal/brainsci/special issues/Neuro Emotion). For citation purposes, cite each article independently as indicated on the article page online and as indicated below: LastName, A.A.; LastName, B.B.; LastName, C.C. Article Title. Journal Name Year, Article Number, Page Range. ISBN 978-3-03921-702-1 (Pbk) ISBN 978-3-03921-703-8 (PDF) c 2019 by the authors. Articles in this book are Open Access and distributed under the Creative Commons Attribution (CC BY) license, which allows users to download, copy and build upon published articles, as long as the author and publisher are properly credited, which ensures maximum dissemination and a wider impact of our publications. The book as a whole is distributed by MDPI under the terms and conditions of the Creative Commons license CC BY-NC-ND. -

On the Scent of Human Olfactory Orbitofrontal Cortex: Meta-Analysis and Comparison to Non-Human Primates
Brain Research Reviews 50 (2005) 287 – 304 www.elsevier.com/locate/brainresrev Review On the scent of human olfactory orbitofrontal cortex: Meta-analysis and comparison to non-human primates Jay A. Gottfrieda,*, David H. Zaldb aDepartment of Neurology and the Cognitive Neurology and Alzheimer’s Disease Center, Northwestern University Feinberg School of Medicine, 320 E. Superior St., Searle 11-453, Chicago, IL 60611, USA bDepartment of Psychology, Vanderbilt University, Nashville, TN 37240, USA Accepted 25 August 2005 Available online 6 October 2005 Abstract It is widely accepted that the orbitofrontal cortex (OFC) represents the main neocortical target of primary olfactory cortex. In non-human primates, the olfactory neocortex is situated along the basal surface of the caudal frontal lobes, encompassing agranular and dysgranular OFC medially and agranular insula laterally, where this latter structure wraps onto the posterior orbital surface. Direct afferent inputs arrive from most primary olfactory areas, including piriform cortex, amygdala, and entorhinal cortex, in the absence of an obligatory thalamic relay. While such findings are almost exclusively derived from animal data, recent cytoarchitectonic studies indicate a close anatomical correspondence between non-human primate and human OFC. Given this cross-species conservation of structure, it has generally been presumed that the olfactory projection area in human OFC occupies the same posterior portions of OFC as seen in non-human primates. This review questions this assumption by providing a critical survey of the localization of primate and human olfactory neocortex. Based on a meta-analysis of human functional neuroimaging studies, the region of human OFC showing the greatest olfactory responsivity appears substantially rostral and in a different cytoarchitectural area than the orbital olfactory regions as defined in the monkey. -

Brainstem and Its Associated Cranial Nerves
Brainstem and its Associated Cranial Nerves Anatomical and Physiological Review By Sara Alenezy With appreciation to Noura AlTawil’s significant efforts Midbrain (Mesencephalon) External Anatomy of Midbrain 1. Crus Cerebri (Also known as Basis Pedunculi or Cerebral Peduncles): Large column of descending “Upper Motor Neuron” fibers that is responsible for movement coordination, which are: a. Frontopontine fibers b. Corticospinal fibers Ventral Surface c. Corticobulbar fibers d. Temporo-pontine fibers 2. Interpeduncular Fossa: Separates the Crus Cerebri from the middle. 3. Nerve: 3rd Cranial Nerve (Oculomotor) emerges from the Interpeduncular fossa. 1. Superior Colliculus: Involved with visual reflexes. Dorsal Surface 2. Inferior Colliculus: Involved with auditory reflexes. 3. Nerve: 4th Cranial Nerve (Trochlear) emerges caudally to the Inferior Colliculus after decussating in the superior medullary velum. Internal Anatomy of Midbrain 1. Superior Colliculus: Nucleus of grey matter that is associated with the Tectospinal Tract (descending) and the Spinotectal Tract (ascending). a. Tectospinal Pathway: turning the head, neck and eyeballs in response to a visual stimuli.1 Level of b. Spinotectal Pathway: turning the head, neck and eyeballs in response to a cutaneous stimuli.2 Superior 2. Oculomotor Nucleus: Situated in the periaqueductal grey matter. Colliculus 3. Red Nucleus: Red mass3 of grey matter situated centrally in the Tegmentum. Involved in motor control (Rubrospinal Tract). 1. Inferior Colliculus: Nucleus of grey matter that is associated with the Tectospinal Tract (descending) and the Spinotectal Tract (ascending). Tectospinal Pathway: turning the head, neck and eyeballs in response to a auditory stimuli. 2. Trochlear Nucleus: Situated in the periaqueductal grey matter. Level of Inferior 3. -

Achems XLII April 19 – 23, 2021 Virtual Meeting Program & Abstracts
AChemS XLII April 19 – 23, 2021 Virtual Meeting Program & Abstracts Monday, April 19, 2021 Monday, April 19, 2021 10:00 - 12:00 PM Welcome & Keynote Lecture Chair(s): Max Fletcher Welcome by AChemS 2021 President. Linda Barlow University of Colorado Anschutz Medical Campus Program Highlights. Max Fletcher The University of Tennessee Health Science Center Awards Ceremony. Nirupa Chaudhari University of Miami KEYNOTE: Mouse Facial Expressions Reflect Emotions and Reveal Subjective Value. Nadine Gogolla Max Planck Institute of Neurobiology Questions & Answers Monday, April 19, 2021 12:00 - 1:00 PM Graduate Students and Postdoc Member Meet & Greet (Graduate Students and Postdocs Only) Chair(s): Jess Kanwal, Kellie Hyde, Kara Fulton Monday, April 19, 2021 1:00 - 3:00 PM Cell Types in Taste Buds and Tentacles 1 Chair(s): Thomas Finger, Sue Kinnamon Cell Types in Taste Buds and Tentacles. Thomas Finger Rocky Mtn. Taste & Smell Ctr. / U. Colo Med Sch This symposium will discuss the current thinking about the diversity of cells within taste buds and chemotactile sensory organs of octopus suckers. While octopus suckers may seem an odd juxtaposition, both taste buds and sucker chemosensory receptor cells share the property of being contact chemosensory organs responsive to sapid chemical cues crucial for eliciting feeding. Both sucker chemotactile sensory organs and taste buds possess different morphological types of receptor cells that correlate with different functional properties. Vertebrate taste buds are classically described as possessing 3 types of elongate taste cells yet recent studies suggest additional cell types exist and raise the question of how to define cell types in any chemoreceptor system. -

Journal of Biomechanical Science and Engineering Researches on Biomechanical Properties and Models of Peripheral Nerves
Bulletin of the JSME Vol.12, No.1, 2017 Journal of Biomechanical Science and Engineering 【Review Paper】 Researches on biomechanical properties and models of peripheral nerves - a review Ming-Shaung JU*, Chou-Ching K. LIN** and Cheng-Tao CHANG* *Department of Mechanical Engineering, National Cheng Kung University 1 University Road, Tainan 701, Taiwan (R.O.C.) E-mail: [email protected] **Department of Neurology College of Medicine and University Hospital National Cheng Kung University 1 University Road, Tainan 701, Taiwan (R.O.C.) Received: 14 December 2016; Revised: 11 February 2017; Accepted: 6 March 2017 Abstract The biomechanics of peripheral nerve plays an important role in physiology of the healthy and pathology of the diseased such as the diabetic neuropathy, the cauda equine compression and the carpal tunnel syndrome. The other application is recent development of neural prostheses. The goal of this paper is to review researches done by members of Taiwanese Society of Biomechanics on the biomechanics of peripheral nerves in the past two decades. First, a brief introduction of the anatomy of peripheral nerve is given. Then experiment designs and mechanical models for testing the nerves are presented. The material properties ranged from linear elastic to nonlinear visco-elastic and scales ranged from organ level to tissue level are reviewed. Implications of these studies to clinical problems will be addressed and the challenges and future perspective on biomechanics of peripheral nerve are discussed. Key words: Biomechanics, Peripheral nerve, Review, Diabetic mellitus, Cauda equine 1. Introduction Nervous system plays an important role in the human body it can coordinate voluntary and involuntary actions of other tissues and sub-systems. -

Role of P2y Receptors in the Spinal-Trigeminal System in Vivo and in Vitro
UNIVERSITÀ DEGLI STUDI DI MILANO Facoltà di Farmacia Dipartimento di Scienze Farmacologiche Corso di Dottorato di Ricerca in Scienze Farmacotossicologiche, Farmacognostiche e Biotecnologie Farmacologiche (XXIII CICLO) Graduate School in Pharmacological Sciences / Scuola di Dottorato in Scienze farmacologiche TESI DI DOTTORATO DI RICERCA PURINERGIC TRANSMISSION IN MIGRAINE: ROLE OF P2Y RECEPTORS IN THE SPINAL-TRIGEMINAL SYSTEM IN VIVO AND IN VITRO BIO/14 Tesi di dottorato di: GIOVANNI VILLA MATRICOLA: R07517 TUTOR: Chiar.ma Prof.ssa Maria Pia ABBRACCHIO CORRELATORE: Dr.ssa Stefania CERUTI COORDINATORE: Chiar.mo Prof. Guido FRANCESCHINI ANNO ACCADEMICO 2009/2010 Index INDEX 1. INTRODUCTION _______________________________ 1 1.1 PAIN AND NOCICEPTION 2 1.1.1 Molecular basis of nociception 3 1.1.2 The trigeminal nerve and the spinal-trigeminal system 4 1.1.3 Role of non-neuronal cells in pain transmission 9 1.2 MIGRAINE 12 1.2.1 Description of the migraine attack 14 1.2.2 How and where does the migraine attack originate? 15 1.2.3 Familial hemiplegic migraine 21 1.2.4 Current and future pharmacological treatment of migraine 24 1.3 THE PURINERGIC SYSTEM 29 1.3.1 Purinergic signalling 30 1.3.2 P2X receptors 32 1.3.3 P2Y receptors 33 1.3.4 Pathophysiological roles of extracellular nucleotides in the nervous system 36 1.4 PURINES AND PAIN 41 1.4.1 Role of P2X receptors in pain transmission 42 1.4.2 Role of P2Y receptors in pain transmission: sensory ganglia 46 1.4.3 Role of P2Y receptors in pain transmission: CNS 48 2. -

The Human Cortical Dental Pain Matrix : Neural Activation Patterns of Tooth Pain Investigated with Fmri
Zurich Open Repository and Archive University of Zurich Main Library Strickhofstrasse 39 CH-8057 Zurich www.zora.uzh.ch Year: 2011 The human cortical dental pain matrix : neural activation patterns of tooth pain investigated with fMRI Brügger, Michael Posted at the Zurich Open Repository and Archive, University of Zurich ZORA URL: https://doi.org/10.5167/uzh-164035 Dissertation Published Version Originally published at: Brügger, Michael. The human cortical dental pain matrix : neural activation patterns of tooth pain investigated with fMRI. 2011, University of Zurich, Faculty of Arts. The Human Cortical Dental Pain Matrix Neural Activation Patterns of Tooth Pain investigated with fMRI Thesis presented to the Faculty of Arts of the University of Zurich for the degree of Doctor of Philosophy by Michael Brügger of Marbach SG Accepted in the spring semester 2009 on the recommendation of Prof. Dr. rer. nat. Lutz Jäncke and Prof. Dr. med. dent. Sandro Palla Zurich, 2011 …the authors "art‐like" interpretation of a human brain under tooth pain… CONTENTS SUMMARY .................................................................................................................................. 6 ZUSAMMENFASSUNG ................................................................................................................ 7 PREFACE ..................................................................................................................................... 9 1. INTRODUCTION ....................................................................................................