Natural and Anthropogenic Dynamics of Vegetation in the Aral Sea Coast
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Conservationally Important Macrophytes in the Bulgarian Stretch of the Danube River and the Near Water Bodies
Conservationally important macrophytes in the Bulgarian stretch of the Danube river and the near water bodies Vladimir Valchev1, Valeri Georgiev1, Daniella Ivanova1, Sonya Tsoneva1, and Georg Janauer2 Keywords: macrophytes, conservation, Danube, Bulgaria Introduction In the course of several projects (Multifunctional Integrated Study Danube / Corridor and Catchment (MIDCC), Developing an electronic database of the macrophytes in Bulgaria, Red Lists of Bulgarian Vascular Plants and Fungi project, and Red Data Book of Bulgaria (new edition project), the macrophytes were studied in the Bulgarian stretch of Danube river and the near water bodies. Nine conservationally important species were found (Euphorbia lucida Waldst. & Kit., Lemna gibba L., Marsilea quadrifolia L., Nymphaea alba L., Nymphoides peltata (S.G. Gmel.) Kuntze, Salvinia natans (L.) All., Thelypteris palustris Schott, Trapa natans L., Utricularia vulgaris L.), which are the subject of this paper. Methods Field survey methods were applied in the period 2002 − 2005. For the inventarization of the plant species a boat, rake and long waders were used. The plants were photographed in the field, and samples for the herbarium SOM were collected. As a taxonomic basis the Field Guide to the Vascular Plants in Bulgaria (KOZHUHAROV 1992) was used. The conservation status is according to the Red Lists of Bulgarian Vascular Plants and Fungi project, which followed the IUCN assessment criteria (IUCN 2001). Bern Convention (CONVENTION ON THE CONSERVATION OF EUROPEAN WILDLIFE AND NATURAL HABITATS 1979), EC Habitats Directive (DIRECTIVE 92/43/EEC ON THE CONSERVATION OF NATURAL HABITATS AND OF WILD FAUNA AND FLORA 1992), and the Bulgarian Biodiversity Protection Law (BIODIVERSITY PROTECTION LAW OF BULGARIA 2002) are also used. -

Download the Full Report Pdf, 2.9 MB
VKM Report 2016:50 Assessment of the risks to Norwegian biodiversity from the import and keeping of aquarium and garden pond plants Opinion of the Panel on Alien Organisms and Trade in Endangered Species (CITES) of the Norwegian Scientific Committee for Food Safety Report from the Norwegian Scientific Committee for Food Safety (VKM) 2016:50 Assessment of the risks to Norwegian biodiversity from the import and keeping of aquarium and garden pond plants Opinion of the Panel on Alien Organisms and Trade in Endangered Species (CITES) of the Norwegian Scientific Committee for Food Safety 01.11.2016 ISBN: 00000-00000 Norwegian Scientific Committee for Food Safety (VKM) Po 4404 Nydalen N – 0403 Oslo Norway Phone: +47 21 62 28 00 Email: [email protected] www.vkm.no www.english.vkm.no Suggested citation: VKM (2016). Assessment of the risks to Norwegian biodiversity from the import and keeping of aquarium and garden pond plants. Scientific Opinion on the on Alien Organisms and Trade in Endangered species of the Norwegian Scientific Committee for Food Safety ISBN: 978-82-8259-240-6, Oslo, Norway. VKM Report 2016:50 Title: Assessment of the risks to Norwegian biodiversity from the import and keeping of aquarium and garden pond plants Authors preparing the draft opinion Hugo de Boer (chair), Maria G. Asmyhr (VKM staff), Hanne H. Grundt, Inga Kjersti Sjøtun, Hans K. Stenøien, Iris Stiers. Assessed and approved The opinion has been assessed and approved by Panel on Alien organisms and Trade in Endangered Species (CITES). Members of the panel are: Vigdis Vandvik (chair), Hugo de Boer, Jan Ove Gjershaug, Kjetil Hindar, Lawrence Kirkendall, Nina Elisabeth Nagy, Anders Nielsen, Eli K. -

Atlas of Freshwater Key Biodiversity Areas in Armenia
Freshwater Ecosystems and Biodiversity of Freshwater ATLAS Key Biodiversity Areas In Armenia Yerevan 2015 Freshwater Ecosystems and Biodiversity: Atlas of Freshwater Key Biodiversity Areas in Armenia © WWF-Armenia, 2015 This document is an output of the regional pilot project in the South Caucasus financially supported by the Ministry of Foreign Affairs of Norway (MFA) and implemented by WWF Lead Authors: Jörg Freyhof – Coordinator of the IUCN SSC Freshwater Fish Red List Authority; Chair for North Africa, Europe and the Middle East, IUCN SSC/WI Freshwater Fish Specialist Group Igor Khorozyan – Georg-August-Universität Göttingen, Germany Georgi Fayvush – Head of Department of GeoBotany and Ecological Physiology, Institute of Botany, National Academy of Sciences Contributing Experts: Alexander Malkhasyan – WWF Armenia Aram Aghasyan – Ministry of Nature Protection Bardukh Gabrielyan – Institute of Zoology, National Academy of Sciences Eleonora Gabrielyan – Institute of Botany, National Academy of Sciences Lusine Margaryan – Yerevan State University Mamikon Ghasabyan – Institute of Zoology, National Academy of Sciences Marina Arakelyan – Yerevan State University Marina Hovhanesyan – Institute of Botany, National Academy of Sciences Mark Kalashyan – Institute of Zoology, National Academy of Sciences Nshan Margaryan – Institute of Zoology, National Academy of Sciences Samvel Pipoyan – Armenian State Pedagogical University Siranush Nanagulyan – Yerevan State University Tatyana Danielyan – Institute of Botany, National Academy of Sciences Vasil Ananyan – WWF Armenia Lead GIS Authors: Giorgi Beruchashvili – WWF Caucasus Programme Office Natia Arobelidze – WWF Caucasus Programme Office Arman Kandaryan – WWF Armenia Coordinating Authors: Maka Bitsadze – WWF Caucasus Programme Office Karen Manvelyan – WWF Armenia Karen Karapetyan – WWF Armenia Freyhof J., Khorozyan I. and Fayvush G. 2015 Freshwater Ecosystems and Biodiversity: Atlas of Freshwater Key Biodiversity Areas in Armenia. -

UPTAKE and DISTRIBUTION of Cr (VI) in P. STRATIOTES L
2014 3rd International Conference on Environment, Chemistry and Biology IPCBEE vol.78 (2014) © (2014) IACSIT Press, Singapore DOI: 10.7763/IPCBEE. 2014. V78. 16 UPTAKE AND DISTRIBUTION OF Cr (VI) IN P. STRATIOTES L. Sufia Irfan1 and Shardendu2 1Biology Department, University of Tabuk, Tabuk-71491, Saudi Arabia, 2Laboratory of Environment and Biotechnology, Department of Botany, Patna Science College, Patna University, Patna 800005, India Abstract. Pistia stratiotes was cultured in various concentration of Cr (VI) solution (10, 20, 35, 50 µM) for 25 days. The trend of bioaccumulation was high in roots than in the shoots of the experimental organism. Cr (VI) toxicity in the plant caused decrease in phytomass and chlorophyll biosynthesis. Metal uptake rate was also directly proportional to the metal concentration in the culture medium. Keywords: biomass, chlorophyll, Cr (VI), Enzyme kinetics, Metal toxicity, Pistia stratiotes 1. Introduction Ecosystem degradation is directly affected by the disposal of sewage, agricultural and industrial pollutants directly in to the aquatic system causing pollution and one of them are, heavy metal pollution. Phytotechnologies involves process of metal removal via physical, biological and biochemical mechanisms taking place in water, biota and suspended solids of the aquatic bodies [1]. Wetland macrophytes function as biofilter agent and their well-developed vascular systems [2] favors the bioaccumulation efficiency [3]. Macrophytes are not only a food and shelter entity for the aquatic organisms [4] but also a reservoir for nutrients and trace metals depending on the plant species metal accumulation ability [5] and the types of metal [6]. Several studies have been performed to explore the efficacy of aquatic macrophytes in assimilation of metals in to the biomass [7], [8] and field studies involving metal distribution and circulation in water, sediment and aquatic biota [9]. -

(Coleoptera: Curculionidae) for the Control of Salvinia
Louisiana State University LSU Digital Commons LSU Doctoral Dissertations Graduate School 2011 Introduction and Establishment of Cyrtobagous salviniae Calder and Sands (Coleoptera: Curculionidae) for the Control of Salvinia minima Baker (Salviniaceae), and Interspecies Interactions Possibly Limiting Successful Control in Louisiana Katherine A. Parys Louisiana State University and Agricultural and Mechanical College Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_dissertations Part of the Entomology Commons Recommended Citation Parys, Katherine A., "Introduction and Establishment of Cyrtobagous salviniae Calder and Sands (Coleoptera: Curculionidae) for the Control of Salvinia minima Baker (Salviniaceae), and Interspecies Interactions Possibly Limiting Successful Control in Louisiana" (2011). LSU Doctoral Dissertations. 1565. https://digitalcommons.lsu.edu/gradschool_dissertations/1565 This Dissertation is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Doctoral Dissertations by an authorized graduate school editor of LSU Digital Commons. For more information, please [email protected]. INTRODUCTION AND ESTABLISHMENT OF CYRTOBAGOUS SALVINIAE CALDER AND SANDS (COLEOPTERA: CURCULIONIDAE) FOR THE CONTROL OF SALVINIA MINIMA BAKER (SALVINIACEAE), AND INTERSPECIES INTERACTIONS POSSIBLY LIMITING SUCCESSFUL CONTROL IN LOUISIANA. A Dissertation Submitted to the Graduate Faculty of the Louisiana State University and Agricultural and Mechanical College in partial fulfillment of the requirements for the degree of Doctor of Philosophy in The Department of Entomology By Katherine A. Parys B.A., University of Rhode Island, 2002 M.S., Clarion University of Pennsylvania, 2004 December 2011 ACKNOWLEDGEMENTS In pursing this Ph.D. I owe many thanks to many people who have supported me throughout this endeavor. -

Water Spangles (Salvinia Minima) ERSS
Water Spangles (Salvinia minima) Ecological Risk Screening Summary U.S. Fish & Wildlife Service, December 2014 Revised, April 2018 Web Version, 8/19/2019 Photo: Kurt Stüber. Licensed under Creative Commons Attribution-Share Alike 3.0 Unported. Available: https://commons.wikimedia.org/wiki/File:Salvinia_minima_1.jpg. (April 2018). 1 Native Range and Status in the United States Native Range GISD (2018) lists Salvinia minima as native to Argentina, Belize, Bolivia, Brazil, Colombia, Cuba, Ecuador, El Salvador, Guatemala, Honduras, Mexico, Nicaragua, Panama, Paraguay, Peru, Puerto Rico, Uruguay, and Venezuela. From Howard Morgan (2018): “Native Range: Central and South America; common and wide-ranging from southern Mexico to northern Argentina and Brazil (Mickel a[n]d Beitel 1988, [Stoltze] 1983). De la Sota (1976) 1 remarked that, in Argentina, the natural range of Salvinia minima could not be precisely determined due to its frequency in the watergarden and aquarium trade.” Status in the United States GISD (2018) lists Salvinia minima as alien, invasive and established in Alabama, Florida, Louisiana, Minnesota, New York, and Texas. Howard Morgan (2018) list Salvinia minima as present in the wild in Alabama (first report in 1982), Arkansas (first report in 1998), California (first report in 2008), Florida (first report in 1930), Georgia (first report in 1936), Idaho (first report in 2004), Louisiana (first report in 1980), Maryland (first report in 1984), Massachusetts (first report in 1992), Mississippi (first report in 1999), New Mexico (first report in 1999), New York (first report in 1990), Ohio (first report in 2017), Oklahoma (first report in 1989), Puerto Rico (first report in 1998), South Carolina (first report in 1997), and Texas (first report in 1992). -

Environmental Science in the Course of Different Levels
THIS PAGE IS BLANK NEW AGE INTERNATIONAL (P) LIMITED, PUBLISHERS New Delhi · Bangalore · Chennai · Cochin · Guwahati · Hyderabad Jalandhar · Kolkata · Lucknow · Mumbai · Ranchi PUBLISHING FOR ONE WORLD Visit us at www.newagepublishers.com Copyright © 2006 New Age International (P) Ltd., Publishers Published by New Age International (P) Ltd., Publishers All rights reserved. No part of this ebook may be reproduced in any form, by photostat, microfilm, xerography, or any other means, or incorporated into any information retrieval system, electronic or mechanical, without the written permission of the publisher. All inquiries should be emailed to [email protected] ISBN (10) : 81-224-2330-2 ISBN (13) : 978-81-224-2330-3 PUBLISHING FOR ONE WORLD NEW AGE INTERNATIONAL (P) LIMITED, PUBLISHERS 4835/24, Ansari Road, Daryaganj, New Delhi - 110002 Visit us at www.newagepublishers.com Education is a process of development which includes the three major activities, teaching, training and instruction. Teaching is social as well as a professional activity. It is science as well as art. Modern education is not in a sphere but it has a long and large area of study. Now a days most part of the world population is facing different problems related with the nature and they are studying the solutions to save the nature and global problems, but on the second hand we even today do not try to understand our local problems related to the nature. So for the awareness of the problems of P nature and pollution the higher education commission has suggested to add the Environmental Science in the course of different levels. -

Compiled and Circulated by Dr. Poulami Adhikary Mukherjee, Assistant Professor, Department of Zoology, Narajole Raj College
COMPILED AND CIRCULATED BY DR. POULAMI ADHIKARY MUKHERJEE, ASSISTANT PROFESSOR, DEPARTMENT OF ZOOLOGY, NARAJOLE RAJ COLLEGE SSoommee CCoommmmuunniittyy CChhaarraacctteerriissttiicc TTeerrmmiinnoollooggyy BBYY DDRR.. PPOOUULLAAMMII AADDHHIIKKAARRYY MMUUKKHHEERRJJEEEE AASSSSIISSTTAANNTT PPRROOFFEESSSSOORR DDEEPPAARRTTMMEENNTT OOFF ZZOOOOLLOOGGYY NNAARRAAJJOOLLEE RRAAJJ CCOOLLLLEEGGEE ZOOLOGY: SEM- I, PAPER- C2T: ECOLOGY, UNIT 3: COMMUNITY COMPILED AND CIRCULATED BY DR. POULAMI ADHIKARY MUKHERJEE, ASSISTANT PROFESSOR, DEPARTMENT OF ZOOLOGY, NARAJOLE RAJ COLLEGE Vertical Stratification: Stratification in the field of ecology refers to the vertical layering of a habitat; the arrangement of vegetation in layers. It classifies the layers (sing. stratum, pl. strata) of vegetation largely according to the different heights to which their plants grow. The individual layers are inhabited by different animal and plant communities (stratozones). The vertical distribution of different species occupying different levels in an ecosystem is called stratification. Trees occupy the topmost vertical layer of a forest, shrubs occupy ZOOLOGY: SEM- I, PAPER- C2T: ECOLOGY, UNIT 3: COMMUNITY COMPILED AND CIRCULATED BY DR. POULAMI ADHIKARY MUKHERJEE, ASSISTANT PROFESSOR, DEPARTMENT OF ZOOLOGY, NARAJOLE RAJ COLLEGE the second layer, and herbs and grasses occupy the bottommost or base layers. Vertical structure in terrestrial plant habitats: The following layers are generally distinguished: forest floor (root and moss layers), herb, shrub, understory and canopy layers. These vegetation layers are primarily determined by the height of their individual plants, the different elements may however have a range of heights. The actual layer is characterised by the height ZOOLOGY: SEM- I, PAPER- C2T: ECOLOGY, UNIT 3: COMMUNITY COMPILED AND CIRCULATED BY DR. POULAMI ADHIKARY MUKHERJEE, ASSISTANT PROFESSOR, DEPARTMENT OF ZOOLOGY, NARAJOLE RAJ COLLEGE range in which the vast majority of photosynthetic organs (predominantly leaves) are found. -

Coastal Habitats
Appendix D – Coastal habitats Rees, S., Drewitt, A. and Cox, J. D1. Habitat variation Coastal habitats dominated by vascular plants occur in both the supralittoral and littoral zones. In the former, dune, shingle and cliff habitats occur above the tidal limit, exposed to the splash/spray of sea water and maritime climate, but only infrequently covered in sea water during storm events. Coastal change is inevitable: even without sea level rise (which has secondary effects by increasing wave and tidal energy), because it is continually shaped by wind, wave and tidal energy and responds through the combined processes of erosion and accretion. In so doing cliffs erode, beaches build and sand, gravel and fine sediments are moved along the coast. In some areas these changes are slow and perhaps less appreciated; in other areas changes are rapid and have a profound influence on both the natural environment and the way we use and manage the coast. By way of example the 60km of the Holderness coast in East Yorkshire has a documented history of erosion stretching back hundreds of years; the southern parts of this coast are still eroding at more than 2 metres per year. The resulting sediment is vital for the development of beaches and intertidal areas, notably the Humber, the Wash and the estuaries of Essex, north Kent and Suffolk. This sediment is also important to other southern North Sea nations as it circulates towards a sink in the Waddenzee coast of Holland and north-west Germany. These processes of change have been underway since at least the last glaciation, and have led to a varied suite of habitats at the coast. -

The Key to Keyhaven
KS4 Geography 111112 Ecosystems Route: Lymington-Yarmouth The Key to Keyhaven STUDENT INTRODUCTION The picture above shows Keyhaven Saltmarshes, which are part of the more extensive salt marsh system which extends along the coast to the east of Lymington. In this resource you are going to learn all about what slat marshes are and how they develop. On your ferry crossing, from Lymington to Yarmouth (or vice versa), you will be able to see the salt marshes for yourself. You will also be learning about their importance, threats to salt marsh ecosystems and what can be done to manage them. What you learn may form part of a case study for your exam, so make sure you pay attention. Introducing Salt Marshes PRE-VISIT Read the following information very carefully – you will then complete a TASKS series of tasks based on what you have read and learnt! Salt marshes are found in coastal environments where sheltered water allows sediment to be deposited and build up over time to create a unique ecosystem. Sheltered water is found behind a spit, and this can provide the perfect conditions for deposition of sediment. Similarly, estuaries, where rivers enter the sea, are areas where large accumulations of sediment which have been carried down by the river can be deposited. Where they develop at river mouths, the water will be brackish – partly salty and partly fresh water). Sediment is deposited and initially builds up to form mud flats. These are exactly what the name suggests – flat areas of mud! They are what is known as intertidal, in that they are covered at high tide and exposed at low tide. -
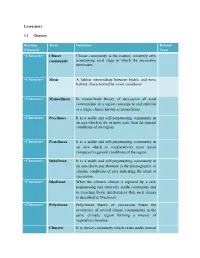
Maintaining Seral Stage in Which the Succession Terminates. a Habitat
Learn more 1.1 Glossary Starting Term Definition Related Character Term <Character> Climax Climax community is the mature, relatively self- community maintaining seral stage in which the succession terminates. <Character> Mesic A habitat intermediate between hydric and xeric habitat, characterized by moist conditions <Character> Monoclimax In monoclimax theory of succession all seral communities in a region converge to and stabilize at a single climax known as monoclimax <Character> Preclimax It is a stable and self-perpetuating community in an area which is dry or more xeric than the general conditions of the region. <Character> Postclimax It is a stable and self-perpetuating community in an area which is comparatively more moist compared to general conditions of the region <Character> Subclimax It is a stable and self-perpetuating community in an area due to peculiarities in the physiographic or edaphic conditions of area indicating the arrest of succession <Character> Disclimax When the climatic climax is replaced by a self- perpetuating and relatively stable community due to recurring biotic interferences then such climax is described as Disclimax. <Character> Polyclimax Polyclimax theory of succession States the occurrence of several climax communities in the same climatic region forming a mosaic of vegetation climaxes. Climatic It is climax community which exists under normal climax climatic conditions in absence of any form of disturbance Topographic It is a self- perpetuating and stable community climax arising due to differences in topography which may give rise to different local micro-climates. Fire climax It is a climax community arising in response to recurrent burning of vegetation which eliminate the fire susceptible species. -

The Vegetation of the Coastal Region of Suriname. Results of the Scientific Expedition to Suriname 1948—49 Botanical Series No
The vegetation of the Coastal Region of Suriname. Results of the scientific expedition to Suriname 1948—49 botanical series No. 1 by J.C. Lindeman (Utrecht) CONTENTS GENERAL PART CHAPTER I. Introduction. 1 ....... Literature 2 Methods 5 ......... Terminology 7 ......... Vernacular 9 names ........ Value of vernacular names 12 The soil. 14 .......... The climate 18 CHAPTER II. AERIAL PHOTOGRAPHS 21 General remarks 21 Difficulties with herbaceous vegetation; illumination effects. 22 results 25 Interpretation ........ and 25 Mangrove swamps ....... Savannas 26 ......... Forests 27 SPECIAL PART CHAPTER III. DESCRIPTION OF THE LANDSCAPE IN THE INVESTIGATED AREAS 28 I. Wia- Transect from Moengo tapoe to the ‘Wiawiabank’ or wia flat 28 The saline coastal belt 29 . articulatus and Machaerium Typha-Cyperus swamp lunatum scrub 31 ........ Third to sixth with Cereus wood ridge , . 31 . Leersia hexandra and 32 swamps Erythrina glauca groves . Second series of ridges and 33 Cyperus giganteus swamps . The old ridges beyond km 9.5 34 II The 35 swaying swamps ....... The oldest and the savannas 37 ridges ..... II. Coronie 38 of the road 38 Brackish North . area . and fresh-water South of the road 39 Ridge complex swamp Third and fourth line 40 Totness 40 III. Nickerie 41 belt and brackish North of the Nickerie Mangrove swamps River 41 Fresh-water area South of the Nickerie River 44 ... IV. Tibiti 45 CHAPTER IV. MANGROVE AND STRAND 46 . ... I. belts the lower the rivers 46 Mangrove along part of . along rivers outside Suriname 48 Mangrove . and other 49 Epiphytes accompanying species ... II. Coastal 50 mangrove ........ Regressing coast 50 Accrescent 50 coast . Accompanying species ....... 53 Mixed forest 54 mangrove ......