Coastal Habitats
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Maintaining Seral Stage in Which the Succession Terminates. a Habitat
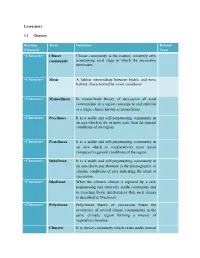
Learn more 1.1 Glossary Starting Term Definition Related Character Term <Character> Climax Climax community is the mature, relatively self- community maintaining seral stage in which the succession terminates. <Character> Mesic A habitat intermediate between hydric and xeric habitat, characterized by moist conditions <Character> Monoclimax In monoclimax theory of succession all seral communities in a region converge to and stabilize at a single climax known as monoclimax <Character> Preclimax It is a stable and self-perpetuating community in an area which is dry or more xeric than the general conditions of the region. <Character> Postclimax It is a stable and self-perpetuating community in an area which is comparatively more moist compared to general conditions of the region <Character> Subclimax It is a stable and self-perpetuating community in an area due to peculiarities in the physiographic or edaphic conditions of area indicating the arrest of succession <Character> Disclimax When the climatic climax is replaced by a self- perpetuating and relatively stable community due to recurring biotic interferences then such climax is described as Disclimax. <Character> Polyclimax Polyclimax theory of succession States the occurrence of several climax communities in the same climatic region forming a mosaic of vegetation climaxes. Climatic It is climax community which exists under normal climax climatic conditions in absence of any form of disturbance Topographic It is a self- perpetuating and stable community climax arising due to differences in topography which may give rise to different local micro-climates. Fire climax It is a climax community arising in response to recurrent burning of vegetation which eliminate the fire susceptible species. -

Plant Communities; Characteristics: a Plant Community Is a Recognizable and Complex Assemblage of Plant Species Which Interact W
CORE COURSE BOTANY-PAPER II- Plant Ecology and Taxonomy (B. Sc. II Semester CBCS 2016) Plant communities; characteristics: A plant community is a recognizable and complex assemblage of plant species which interact with each other as well as with the elements of their environment and is distinct from adjacent assemblages. A plant community is not a static entity: rather it may vary in appearance and species composition from location to location and also over time. What makes each of these communities distinguishable to us is its general physiognomy or physical structure. This overall appearance is created by the particular species present, as well as their size, abundance, and distribution relative to one another. Dominant species, those whose presence most influences the community environment and composition, are often the largest or the most abundant and may be a single species or several codominant species. Dominance may also be sociologic, expressed in the form of allelopathogens, chemical compounds manufactured by some plants that inhibit the growth and development of other species and/or seedlings of the same species within a certain distance. Plant communities may occur as relatively obvious ribbons across a landscape, such as the lush green path of a river through the desert. More often, however, adjacent communities interdigitate, their boundaries less distinguishable. Ecotones are areas where adjacent plant communities overlap with, transition, and grade into one another and have a unique set of characteristics which are defined spatially, temporally, and by diverse interactions among the adjacent communities. Ecotones vary in size and species composition, containing elements of each of the bordering communities. -

ZOOLOGY Principles of Ecology Community
Paper No. : 12 Principles of Ecology Module : 20 Community: Community characteristics, types of biodiversity, diversity index, abundance, species richness, vertical and horizontal stratification: Part IV Development Team Principal Investigator: Prof. Neeta Sehgal Head, Department of Zoology, University of Delhi Co-Principal Investigator: Prof. D.K. Singh Department of Zoology, University of Delhi Paper Coordinator: Prof. D.K. Singh Department of Zoology, University of Delhi Content Writer: Dr. Haren Ram Chiary and Dr. Kapinder Kirori Mal College, University of Delhi Content Reviewer: Prof. K.S. Rao Department of Botany, University of Delhi 1 Principles of Ecology ZOOLOGY Community: Community characteristics, types of biodiversity, diversity index, abundance, species richness, vertical and horizontal stratification: Part IV Description of Module Subject Name ZOOLOGY Paper Name Zool 12, Principles of Ecology Module Name/Title Community Module Id M20, Community characteristics, types of biodiversity, diversity index, abundance, species richness, vertical and horizontal stratification : Part-IV Keywords Succession, Primary succession, secondary succession, Sera, Climax community, Hydrosere, Lithosere, theories of climax community Contents 1. Learning Objective 2. Introduction 3. History of study of succession 4. Ecological succession and types: Primary and secondary succession 5. Stages of Primary and secondary succession 6. Process of succession in Hydrosere 7. Process of succession in Lithosere 8. Theories of climax community 9. Summary -

IB Geography Knowledge Audit – Ecosystems and Human Activity
Name IB Geography Knowledge Audit – Ecosystems and Human Activity The concept of the ecosystem Terms and definitions Define the term ecosystem. Be aware of variations in ecosystem scale ranging from micro-scales (niches) to macroscales (biomes). Be able to define ecosystems in terms of inputs, flows, stores and outputs. The components of an ecosystem Biotic and Abiotic Components Demonstrate an awareness of biotic (plants, animals, soil, bacteria, fungi) components. Demonstrate an awareness of abiotic (water, air, minerals, nutrients, light) components. Demonstrate an awareness of how the biotic and abiotic components interrelate to form a natural functioning system Take into account other contributory factors of acidity, temperature, humidity and wind. Links between the various components Understand the concept of dynamic equilibrium. Understand the concept of food webs. Understand the concept of food chains. Demonstrate an awareness of the complexity of links between biotic and abiotic components and how changes to one or more of the components can disturb the equilibrium of the system, especially as a result of human activity. Understand how positive and negative feedback contribute to the stability of the system. Fragility, vulnerability and resilience of the system Understand that changes to ecosystems may be temporary (where recovery may occur leading to the re-establishment of the system) or permanent, leading to modification of the system. Processes operative within an ecosystem Energy flows Understand how energy enters the system through photosynthesis. Understand how energy is transferred from producers (autotrophs) to consumers (herbivores, carnivores and detritovores). Page 1 of 3 http://www.geographyalltheway.com/ Understand the changes in energy and biomass from one level to the next. -

MU Ecological Succession
ECOLOGICAL SUCCESSION Prepared by Dr. Rukhshana Parveen Assistant Professor, Department of Botany Gautum Buddha Mahila College, Gaya Magadh University, Bodhgaya Ecological Succession is also called as Plant Succession or Biotic succession. Hult (1885) used the term” succession”. The authentic studies on succession were started in America by Cowles (1899) and Clements (1907). The occurrence of relatively definite sequence of communities over a long period of time in the same area resulting in establishment of stable community is called ecological succession. It allows new areas to be colonized and damaged ecosystems to be recolonized, so organisms can adapt to the changes in the environment and continue to survive. Major types of ecological succession. 1. Primary Succession- When the succession starts from barren area such as bare rock or open water. It is called primary succession. Figure1:- Primary succession. 2. Secondary Succession- Secondary succession occurs when the primary ecosystem gets destroyed by fire or any other agent. It gets recolonized after the destruction. This is known as secondary ecological succession. Figure2:- Secondary succession. 3. Autogenic succession:- After succession has begun, Its vegetation itself cause its own replacement by new communities is called Autogenic succession 4. Cyclic Succession: - This refers to repeated occurrence of certain stages of succession. 5. Allogenic succession:- When the replacement of existing community is caused by any other external condition and not by existing vegetation itself. This is called allogenic succession. 6. Autotropoic succession:- It is characterised by early and continued dominance of autotrophic organism called green plants. 7. Heterotropic succession:- It is characterised by early dominance of heterotrophs such as bacteria, actinomycetes, fungi and animal. -

DU Msc Environmental Studies Topic:- DU J19 MSC ES
DU MSc Environmental Studies Topic:- DU_J19_MSC_ES 1) What are chionophiles? [Question ID = 12617] 1. Organisms that can tolerate excessive salinity [Option ID = 20466] 2. Organisms that can survive very cold conditions [Option ID = 20468] 3. Organisms that can survive arid conditions [Option ID = 20465] 4. Organisms that can survive in space [Option ID = 20467] Correct Answer :- Organisms that can survive very cold conditions [Option ID = 20468] 2) Which of the following taxa has the most number of species? [Question ID = 12538] 1. Insects [Option ID = 20152] 2. Amphibians [Option ID = 20149] 3. Mammals [Option ID = 20150] 4. Plants [Option ID = 20151] Correct Answer :- Insects [Option ID = 20152] 3) Which of the following characteristics are correct for differentiating crocodiles and alligators? Choose the right combination of characters. i. Crocodiles have V-shaped snout and alligators have U-shaped snout ii. Crocodiles have U-shaped snout and alligators have V-shaped snout iii. Crocodiles are generally slower than alligators iv. Fully grown alligators are generally larger in size than fully grown crocodiles v. Crocodiles are circumglobally distributed and alligators are found only in America and China [Question ID = 12625] 1. ii, iv [Option ID = 20500] 2. i, iii, v [Option ID = 20498] 3. ii, iii, v [Option ID = 20499] 4. i, iii,iv [Option ID = 20497] Correct Answer :- i, iii, v [Option ID = 20498] 4) Which of the following types of sewage treatment is properly matched? [Question ID = 12559] 1. Primary - biological [Option ID = 20233] 2. Advanced - physical and chemical [Option ID = 20235] 3. Secondary - mechanical process [Option ID = 20234] 4. Secondary - chemical process [Option ID = 20236] Correct Answer :- Advanced - physical and chemical [Option ID = 20235] 5) Which of the following is most useful for combating virus infection? [Question ID = 12543] 1. -

Natural and Anthropogenic Dynamics of Vegetation in the Aral Sea Coast
American Journal of Environmental Protect ion 2015; 4(3-1): 136-142 Published online June 23, 2015 (http://www.sciencepublishinggroup.com/j/ajep) doi: 10.11648/j.ajep.s.2015040301.31 ISSN: 2328-5680 (Print); ISSN: 2328-5699 (Online) Natural and Anthropogenic Dynamics of Vegetation in the Aral Sea Coast Dimeyeva Liliya Institute of Botany & Phytointroduction /Ministry of Education & Science, Almaty, Republic of Kazakhstan Email address: [email protected] To cite this article: Dimeyeva Liliya. Natural and Anthropogenic Dynamics of Vegetation in the Aral Sea Coast. American Journal of Environmental Protection . Special Issue: Applied Ecology: Problems, Innovations. Vol. 4, No. 3-1, 2015, pp. 136-142. doi: 10.11648/j.ajep.s.2015040301.31 Abstract: Natural dynamics (primary successions) is studied in the dry seabed of the Aral Sea. Long-term studies of vegetation have identified three types of primary successions: psammosere, halosere and potamosere (sere of shrubby riparian vegetation). They differ by soil texture and salinity, patterns of temporal dynamics, and stages, selected on a basis of ecological-physiognomic features of dominant plants. Late seral stages were identified for succession types: psammophytic shrub ( Calligonum spp, Astragalus brachypus , etc.) for psammosere; haloxerophytic and xerophytic dwarf semishrubs ( Anabasis salsa, Artemisia pauciflora, A. terrae-albae ) for halosere. There is a change of a dominant plant and succession dynamics in late seral stages in potamosere ( Tamarix spp. → Calligonum spp, Haloxylon aphyllum , Artemisia terrae-albae ). Anthropogenic dynamics of vegetation (secondary successions) depends on factors of disturbance. There is a set of anthropogenic factors causing degradation of vegetation cover: (1) agricultural: overgrazing, haymaking, plowing, clearing trees and shrubs; (2) linear structures (paved and dirt roads); (3) water management: construction and operation of hydraulic structures, fluctuation in river runoff and the sea level, disturbance in the natural flooding regime; (4) fires; (5) recreations. -

The Non-Marine Mollusca of the Western Isles
ARCHAEOLOGY IN THE WESTERN ISLES: THE MOLLUSCAN EVIDENCE Matt Law PhD in Archaeology School of History, Archaeology and Religion, Cardiff University June 2017 SUMMARY Using assemblages of marine and non-marine mollusc shells from recent excavations in the Western Isles of Scotland, with reference to previously published studies, this thesis contributes to an enhanced understanding of the cultural palaeoecology of insular societies. Chaoter 1 sets out the topics that will be covered in this thesis. Chapter 2 introduces the methods and principles that drive molluscan analysis; Chapter 3 outlines the natural history of the Western Isles; and Chapter 4 the archaeology. Previous work on molluscs from the islands are summarised in Chapter 5, and emergent themes identified. Chapter 6 presents the results of analyses of new non-marine molluscan assemblages from 9 sites, ranging in date from the Mesolithic to the Norse period. Comparative data collected from a transect of samples for modern snails are also presented, along with a statistical meta-analysis of the data. Chapter 7 presents the results of marine shell analyses from 4 sites, ranging in date from the Early Bronze Age to the Norse period. The results are discussed in terms of their regional and wider significance in Chapter 8, and the thesis concluded in Chapter 9. Studying non-marine and marine molluscs from a wide range of sites across the islands has made important contributions to the archaeology of the Western Isles. The movement of new species of snail into and across the islands emphasises the connectedness of prehistoric communities across wider social networks on the Atlantic coast of Europe. -

Unit 7 - Week 6 Communit
Wild Life Ecology - - Unit 7 - Week 6_Communit... https://onlinecourses-archive.nptel.ac.in/noc19_... X [email protected] ▼ Courses » Wild Life Ecology Announcements Course Ask a Question Progress FAQ Unit 7 - Week 6_Community Ecology Register for Certification exam Assignment-6 The due date for submitting this assignment has passed. Course As per our records you have not submitted this Due on 2019-03-13, 23:59 IST. outline assignment. How to access 1) Which of these is not a characteristic of pioneer species 2 points the portal ability to grow on bare rocks Week 1 - Introduction ability to tolerate extreme temperatures large size Week 2 - Ecological short life span structure No, the answer is incorrect. Score: 0 Week 3_Ecological Accepted Answers: Interactions large size Week 2) A climax caused by wildfires is an example of 2 points 4_Ecological Energetics climatic climax edaphic climax Week 5_Population disclimax Ecology catastrophic climax Week No, the answer is incorrect. 6_Community Score: 0 Ecology Accepted Answers: Lecture catastrophic climax 16_Community nature and 3) Which of these is correct? 2 points parameters Lecture Fundamental niche > Realised niche 17_Community Fundamental niche = Realised niche changes and ecological Fundamental niche < Realised niche succession a or b Lecture 18_Community No,© 2014 the NPTELanswer - isPrivacy incorrect. & Terms - Honor Code - FAQs - organisation Score: 0 A project of In association with Quiz : Accepted Answers: Assignment-6 a or b Wild Life 4) Importance value varies from Funded by 2 points Ecology : 1 of 3 Wednesday 12 June 2019 12:51 PM Wild Life Ecology - - Unit 7 - Week 6_Communit... https://onlinecourses-archive.nptel.ac.in/noc19_.. -

Fieldwork Day: Ecosystems Succession Specification: AQA Level: AS
Fieldwork Day: Ecosystems Succession Specification: AQA Level: AS Learning Objectives: • To understand how vegetation in an ecosystem changes over time leading to a climatic climax • Consider which factors are causing this change in vegetation Geographical fieldwork investigation skills • Develop an understanding of setting up an investigation with aims and testing hypotheses • Consider different data collection techniques and sampling strategies which could be used • Understand different ways to present and analyse data Learning Outcomes: All students will be able to: • Observe the changes in vegetation within a successional environment • Collect data on the vegetation, soil and micro-climate within this environment • Outline the fieldwork aims and the hypotheses being tested • Use at least two different techniques to present data including kite diagrams and pick out trends shown using these techniques • Carry out one form of statistical analysis to test on original hypothesis Most students will be able to: • Justify the fieldwork techniques used during the day and outline why some techniques and sampling strategies were used rather than others • Explain what factors are causing changes in vegetation over time in this environment • Pick out anomalies in the data collected and suggest reasons for them • Use GIS and other ICT to help support the investigation process Some students will be able to: • suggest ways the investigation and data collection methods could be improved • suggest specific factors within this unique environment lead -

Characteristics of Hydrosere
Ecological Succession Continued… Depending upon the nature of the habitat on which the plant succession begins seven types of seres may be distinguished: 1. Hydrosere: Hydrosere or Hydrarch succession occurs in a pond and its community are converted into a land community. Characteristics of Hydrosere: In the initial stage, phytoplankton(cyanobacteria), green algae (Spirogyra,Oedogonium) are the pioneer colonizers. They are consumed by zooplankton (protozoans as Amoeba, Euglena, Paramecium etc), fish such as sun fish, blue gill fish etc. Gradually these organisms die and increase the content of dead organic matter in the pond. This is then utilized by bacteria and fungi, and minerals are released after decomposition. Nutrient rich mud supports rooted hydrophytes growth as Elodea, Hydrilla, Ceratophyllum etc. in the shallow water zone. This submerged stage is also inhabited by animals such as may flies. Dragon flies etc and Crustaceans as Daphnia, Cypris, Cyclops etc. The hydrophytes die and decomposed by microorganisms and thus release nutrients. Due to silting, water depth is reduced and at the margin of pond grows rooted floating vegetation. Example- Nelumbo nucifera, Monochoria, Trapa etc. In floating stage faunal living space is increased and diversified. Example- frogs, salamander, hydra, diving beetles etc inhabit such conditions. Some turtles and snake also invade the pond. Gradually, the water depth decreases due to water evaporation and organic matter decomposition. Free floating plants as Lemna, Azolla, Pistia, Spirodella, Wolffia etc increase in number as availability of high nutrient is there. When these die, they build up the pond ecosystem, resulting in further build-up of the substratum. Pond becomes a Swampy ecosystem. -

Dynamics : Succession Ecological Succession Is the Steady and Gradual Change in a Species of a Given Area with Respect to the Changing Environment
Dynamics : Succession Ecological succession is the steady and gradual change in a species of a given area with respect to the changing environment. It is a predictable change and is an inevitable process of nature as all the biotic components have to keep up with the changes in our environment. The ultimate aim of this process is to reach equilibrium in the ecosystem. The community that achieves this aim is called a climax community. In an attempt to reach this equilibrium, some species increase in number while some other decreases. In an area, the sequence of communities that undergo changes is called sere. Thus, each community that changes is called a seral stage or seral community. All the communities that we observe today around us have undergone succession over the period of time since their existence. Thus, we can say that evolution is a process that has taken place simultaneously along with that of ecological succession. Also, the initiation of life on earth can be considered to be a result of this succession process. If we consider an area where life starts from scratch by the process of succession, it is known as primary succession. However, if life starts at a place after the area has lost all the life forms existing there, the process is called secondary succession. It is obvious that primary succession is a rather slow process as life has to start from nothing whereas secondary succession is faster because it starts at a place which had already supported life before. Moreover, the first species that comes into existence during primary succession is known as pioneer species.