Open Myers THESIS.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Bwsr Featured Plant Minnesota's Milkweeds
BWSR FEATURED PLANT MINNESOTA’S MILKWEEDS Publication Date: 6‐1‐13 Milkweeds play a key role in wetlands, prairies, savannas and forests in Minnesota. The genus (Asclepias) is particularly important as a nectar and larval food source for a wide range of insect species. The best known example is the monarch butterfly whose larvae appear to feed only on milkweeds. Milkweeds have a unique pollination mechanism where pollen grains are enclosed in waxy sacs called “pollina” that attach to the legs of butterflies, moths, bees, ants and wasps and are then deposited in another milkweed flower if they step into a specialized anther opening. Most milkweeds are toxic to vertebrate herbivores due to cardiac glycosides that are in their plant cells. In addition to supporting insect populations, Butterfly Milkweed milkweeds also provide other landscape benefits due to their extensive root systems (sometimes deep roots, sometimes horizontal) that Photos by Dave Hanson decrease compaction, add organic material to the soil and improve unless otherwise stated water infiltration. Common milkweed is probably the best known milkweed species as it is found in all counties of the state and was included on some county prohibited noxious weed lists. The species was considered a common agricultural weed as its extensive root network made it difficult to remove from agricultural fields with cultivators. Now the species is effectively removed from genetically modified corn and soybean fields that are sprayed with herbicide. This practice has contributed to significant declines in milkweed species, with an estimated 58% decline in the Midwest between 1999 and 2010 and a corresponding 81% decline in monarch butterfly production (Pleasants & Oberhauser, 2013). -

Field Release of the Leaf-Feeding Moth, Hypena Opulenta (Christoph)
United States Department of Field release of the leaf-feeding Agriculture moth, Hypena opulenta Marketing and Regulatory (Christoph) (Lepidoptera: Programs Noctuidae), for classical Animal and Plant Health Inspection biological control of swallow- Service worts, Vincetoxicum nigrum (L.) Moench and V. rossicum (Kleopow) Barbarich (Gentianales: Apocynaceae), in the contiguous United States. Final Environmental Assessment, August 2017 Field release of the leaf-feeding moth, Hypena opulenta (Christoph) (Lepidoptera: Noctuidae), for classical biological control of swallow-worts, Vincetoxicum nigrum (L.) Moench and V. rossicum (Kleopow) Barbarich (Gentianales: Apocynaceae), in the contiguous United States. Final Environmental Assessment, August 2017 Agency Contact: Colin D. Stewart, Assistant Director Pests, Pathogens, and Biocontrol Permits Plant Protection and Quarantine Animal and Plant Health Inspection Service U.S. Department of Agriculture 4700 River Rd., Unit 133 Riverdale, MD 20737 Non-Discrimination Policy The U.S. Department of Agriculture (USDA) prohibits discrimination against its customers, employees, and applicants for employment on the bases of race, color, national origin, age, disability, sex, gender identity, religion, reprisal, and where applicable, political beliefs, marital status, familial or parental status, sexual orientation, or all or part of an individual's income is derived from any public assistance program, or protected genetic information in employment or in any program or activity conducted or funded by the Department. (Not all prohibited bases will apply to all programs and/or employment activities.) To File an Employment Complaint If you wish to file an employment complaint, you must contact your agency's EEO Counselor (PDF) within 45 days of the date of the alleged discriminatory act, event, or in the case of a personnel action. -

Natural Communities of Michigan: Classification and Description
Natural Communities of Michigan: Classification and Description Prepared by: Michael A. Kost, Dennis A. Albert, Joshua G. Cohen, Bradford S. Slaughter, Rebecca K. Schillo, Christopher R. Weber, and Kim A. Chapman Michigan Natural Features Inventory P.O. Box 13036 Lansing, MI 48901-3036 For: Michigan Department of Natural Resources Wildlife Division and Forest, Mineral and Fire Management Division September 30, 2007 Report Number 2007-21 Version 1.2 Last Updated: July 9, 2010 Suggested Citation: Kost, M.A., D.A. Albert, J.G. Cohen, B.S. Slaughter, R.K. Schillo, C.R. Weber, and K.A. Chapman. 2007. Natural Communities of Michigan: Classification and Description. Michigan Natural Features Inventory, Report Number 2007-21, Lansing, MI. 314 pp. Copyright 2007 Michigan State University Board of Trustees. Michigan State University Extension programs and materials are open to all without regard to race, color, national origin, gender, religion, age, disability, political beliefs, sexual orientation, marital status or family status. Cover photos: Top left, Dry Sand Prairie at Indian Lake, Newaygo County (M. Kost); top right, Limestone Bedrock Lakeshore, Summer Island, Delta County (J. Cohen); lower left, Muskeg, Luce County (J. Cohen); and lower right, Mesic Northern Forest as a matrix natural community, Porcupine Mountains Wilderness State Park, Ontonagon County (M. Kost). Acknowledgements We thank the Michigan Department of Natural Resources Wildlife Division and Forest, Mineral, and Fire Management Division for funding this effort to classify and describe the natural communities of Michigan. This work relied heavily on data collected by many present and former Michigan Natural Features Inventory (MNFI) field scientists and collaborators, including members of the Michigan Natural Areas Council. -

Identification of Milkweeds (Asclepias, Family Apocynaceae) in Texas
Identification of Milkweeds (Asclepias, Family Apocynaceae) in Texas Texas milkweed (Asclepias texana), courtesy Bill Carr Compiled by Jason Singhurst and Ben Hutchins [email protected] [email protected] Texas Parks and Wildlife Department Austin, Texas and Walter C. Holmes [email protected] Department of Biology Baylor University Waco, Texas Identification of Milkweeds (Asclepias, Family Apocynaceae) in Texas Created in partnership with the Lady Bird Johnson Wildflower Center Design and layout by Elishea Smith Compiled by Jason Singhurst and Ben Hutchins [email protected] [email protected] Texas Parks and Wildlife Department Austin, Texas and Walter C. Holmes [email protected] Department of Biology Baylor University Waco, Texas Introduction This document has been produced to serve as a quick guide to the identification of milkweeds (Asclepias spp.) in Texas. For the species listed in Table 1 below, basic information such as range (in this case county distribution), habitat, and key identification characteristics accompany a photograph of each species. This information comes from a variety of sources that includes the Manual of the Vascular Flora of Texas, Biota of North America Project, knowledge of the authors, and various other publications (cited in the text). All photographs are used with permission and are fully credited to the copyright holder and/or originator. Other items, but in particular scientific publications, traditionally do not require permissions, but only citations to the author(s) if used for scientific and/or nonprofit purposes. Names, both common and scientific, follow those in USDA NRCS (2015). When identifying milkweeds in the field, attention should be focused on the distinguishing characteristics listed for each species. -

Hybridization Between Asclepias Purpurascens and Asclepias Syriaca (Apocynaceae): a Cause for Concern?1
Journal of the Torrey Botanical Society 146(4): 278–290, 2019. Hybridization between Asclepias purpurascens and Asclepias syriaca (Apocynaceae): A cause for concern?1 Steven B. Broyles2,4 and Geordie Elkins3 2Biological Sciences Department, SUNY Cortland, Cortland, NY 13045 and 3Highstead, 127 Lonetown Road, Redding, CT 06896 Abstract. Rare plant species can be at risk of hybridization, reduced genetic variation, and genetic assimilation when a numerically abundant congener co-occurs in or invades their habitat. We investigated hybridization between a species in decline, Asclepias purpurascens L., and its common and widespread congener, Asclepias syriaca L. A total of 40 morphological traits were measured on 60 flowering plants from a mixed population in Connecticut. Cluster and principal component analyses identified two distinct clusters of parental species taxa and a third cluster representing putative hybrids. Although leaf traits of putative hybrids were more similar to A. syriaca, floral traits and the morphological space on the principal component analysis were more similar to A. purpurascens. This suggests that the population contains a mix of F1 and advanced generation hybrids with the possibility of introgression into A. purpurascens. Although putative hybrids are intermediate for most traits, pollen counts reveal reduced fertility of presumed hybrid that might influence mating behaviors and increase the likelihood of backcrossing with A. purpurascens. We suggest a combination of morphological traits that better identify the two parental species as well as hybrids in the field. Conservationists should consider options of managing A. syriaca where A. purpurascens needs protection from competition to prevent genetic assimilation of the latter. Key words: Asclepias, conservation, hybridization Angiosperms abound with natural and anthro- 2015). -
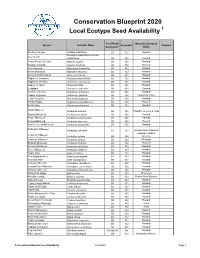
Local Ecotype Seed Species Available
Conservation Blueprint 2020 Local Ecotype Seed Availability 1 Seed Origin Nebraska County of Species Scientific Name Ecoregion Request Generation 2 Origin Western Yarrow Achillea millefolium G0 332 Howard Acmispon americanus or Lotus Deer Vetch unifoliolatus G0 332 Howard Rough Purple Gerardia Agalinis aspera G0 332 Howard Slender Gerardia Agalinis tenuifolia G0 332 Howard Anise Hyssop Agastache foeniculum G2 332 Howard White Snakeroot Ageratina altissima G0 332 Howard Wild Onion/Wild Garlic Allium canadense G0 332 Howard Ragweed, Common Ambrosia artemisiifolia G0 332 Howard Ragweed, Western Ambrosia psilostachya G0 332 Howard Ragweed, Giant Ambrosia trifida G0 332 Howard Leadplant Amorpha canescens G0 332 Howard Caroline Anemone Anemone caroliniana G0 332 Howard Candle Anemone Anemone cylindrica G0 332 Howard & Colfax Field Pussytoes Antennaria neglecta G0 332 Howard Prickly Poppy Argemone polyanthemos G0 332 Howard White Sage Artemisia ludoviciana G0 332 Howard Sand Milkweed Asclepias arenaria G0 332 Howard, Greeley & Loup Swamp Milkweed Asclepias incarnata G1 332 Howard Purple Milkweed Asclepias purpurascens G1 251 Kansas Showy Milkweed Asclepias speciosa G1 332 Howard Narrow-leaved Milkweed Asclepias stenophylla G0 332 Howard Sullivant's Milkweed Asclepias sullivantii G1 332 Howard, Knox & Merrick Howard, Colfax & Common Milkweed Asclepias syriaca G0 332 Greeley Common Milkweed Asclepias syriaca G0 222 Michigan Butterfly Milkweed Asclepias tuberosa G1 332 Howard Whorled Milkweed Asclepias verticillata G0 332 Howard Green Milkweed -

Milkweed and Monarchs
OHIO DEPARTMENT OF NATURAL RESOURCES DIVISION OF WILDLIFE MILKWEEDS AND MONARCHS Acknowledgments Table of Contents We thank Dr. David Horn, past president of the Ohio Lepidopterists, 03 MONARCH LIFE CYCLE for his thoughtful review of this publication. Our appreciation goes 04 MONARCH MIGRATION to the Ohio Lepidopterists, and Monarch Watch. These organizations work tirelessly to promote the conservation of butterflies and moths. 05 PROBLEMS & DECLINE COVER PHOTO BY KELLY NELSON 06 MILKWEEDS 07 OTHER MILKWEED SPECIALISTS 08 MONARCH NURSERY GARDEN Introduction 09 FIVE EXCELLENT MILKWEEDS Text and photos by Jim McCormac, Ohio Division of Wildlife, unless otherwise stated. 10 SUPERB MONARCH NECTAR SOURCES The Monarch butterfly (Danaus plexippus) is one of North Amer- ica’s most iconic insects. The gorgeous golden-brown and black but- terfly is probably the most celebrated insect on the continent, and the migration of the eastern population is conspicuous and spectacular. Southbound Monarchs can appear anywhere, even in highly urban- ized locales, and the butterflies often use backyard gardens as way sta- tions. Occasionally a resting swarm of hundreds or even thousands of butterflies is encountered. The spectacle of trees dripping with living leaves of butterflies is unlikely to be forgotten. PHOTO BY CHRIS FROST A Pictorial Journey From Caterpillar to Chrysalis to Butterfly PHOTOS BY STEVEN RUSSEL SMITH Monarch Butterfly Life Cycle Like all species in the order Lepidoptera (moths and butterflies), soon hatch. The caterpillars begin eating the milkweed foliage, and Monarchs engage in complete metamorphosis. This term indicates grow rapidly. The growth process involves five molts where the cater- that there are four parts to the life cycle: egg, caterpillar, pupa, and pillar sheds its skin and emerges as a larger animal. -

Native Plants & Pollinators
Native Plants & Pollinators What is a “Native” plant? A plant that is a part of the balance of nature that has developed over hundreds or thousands of years in a particular region or ecosystem. What is Ecotype? “Ecotype” refers to a genetically unique population of plants adapted to a local environment. This matters when it comes to foliage, flowering, seed set, seed germination and overall plant performance. What is Nectar? Nectar is a sweet liquid secreted by plants to attract pollinators. It is an important food source for butterflies, bees and hummingbirds. What is Pollen? Pollen carries cells which enable fertilization of plants, thus enabling the plant to reproduce. Examples of Pollinators: Bees, butterflies, beetles, flies, as well as some birds and bats are pollinators. About 12% of the world’s flowering plants are wind-pollinated, including grasses, cereal crops, and many trees. Cross-pollination Self-pollination Why is Pollination So Important? Pollination is mutually beneficial to plants AND to pollinators. Pollination results in the production of seeds and is necessary for many plants to reproduce. Why is Pollination So Important? (cont’d) Pollinators receive nectar and/or pollen rewards from the flowers they visit. Sugary nectar provides pollinators with carbohydrates, while pollen offers proteins, fats, vitamins, minerals, and necessary phytochemicals. Why Do Humans Need Pollinators? Pollinators are necessary for three- quarters of our major food crops. Without pollinators, our diets would be severely limited, and it would be more difficult to acquire the variety of vitamins and minerals we need to stay healthy. Go Native! Add Native Plants Pollinators have evolved with native plants, which often need less water than ornamentals. -

Biotic Inventory and Analysis of the Kettle Moraine State Forest a Baseline Inventory and Analysis of Natural Communities, Rare Plants, and Animals
Biotic Inventory and Analysis of the Kettle Moraine State Forest A Baseline Inventory and Analysis of Natural Communities, Rare Plants, and Animals June 2010 Natural Heritage Inventory Program Bureau of Endangered Resources Department of Natural Resources P.O. Box 7921 PUBL ER-821 2010 Kettle Moraine State Forest - 1 - Cover Photos (Clockwise from top left): Oak Woodland at Kettle Moraine Oak Opening SNA. Photo by Drew Feldkirchner, WDNR; prairie milkweed (Asclepias sullivantii). Photo by Ryan O’Connor, WDNR; Ephemeral Pond on the KMSF. Photo by Ryan O’Connor, WDNR; Northern Ribbon Snake (Thamnophis sauritus). Ohio DNR. Copies of this report can be obtained by writing to the Bureau of Endangered Resources at the address on the front cover. This publication is available in alternative format (large print, Braille, audio tape, etc) upon request. Please call (608-266-7012) for more information. The Wisconsin Department of Natural Resources provides equal opportunity in its employment, programs, services, and functions under an Affirmative Action Plan. If you have any questions, please write to Equal Opportunity Office, Department of Interior, Washington, D.C. 20240. Kettle Moraine State Forest - 2 - Biotic Inventory and Analysis of the Kettle Moraine State Forest A Baseline Inventory and Analysis of Natural Communities, Rare Plants, and Animals Primary Authors: Terrell Hyde, Christina Isenring, Ryan O’Connor, Amy Staffen, Richard Staffen Natural Heritage Inventory Program Bureau of Endangered Resources Department of Natural Resources P.O. -

Floral Glands in Asclepiads: Structure, Diversity and Evolution
Acta Botanica Brasilica - 31(3): 477-502. July-September 2017. doi: 10.1590/0102-33062016abb0432 Review Floral glands in asclepiads: structure, diversity and evolution Diego Demarco1 Received: December 7, 2016 Accepted: February 24, 2017 . ABSTRACT Species of Apocynaceae stand out among angiosperms in having very complex fl owers, especially those of asclepiads, which belong to the most derived subfamily (Asclepiadoideae). Th ese fl owers are known to represent the highest degree of fl oral synorganization of the eudicots, and are comparable only to orchids. Th is morphological complexity may also be understood by observing their glands. Asclepiads have several protective and nuptial secretory structures. Th eir highly specifi c and specialized pollination systems are associated with the great diversity of glands found in their fl owers. Th is review gathers data regarding all types of fl oral glands described for asclepiads and adds three new types (glandular trichome, secretory idioblast and obturator), for a total of 13 types of glands. Some of the species reported here may have dozens of glands of up to 11 types on a single fl ower, corresponding to the largest diversity of glands recorded to date for a single structure. Keywords: anatomy, Apocynaceae, Asclepiadoideae, diversity, evolution, fl ower, secretory structures considering its most derived subfamily Asclepiadoideae. Introduction Th e close relationship between the former families Apocynaceae and Asclepiadaceae has always been recognized Apocynaceae is an extremely diverse family in since its establishment as “Apocineae” by Jussieu (1789). morphological terms, represented by trees, shrubs, herbs and climbers, with single leaves usually opposite, rarely Although Brown (1810) divided it into two families and alternate or whorled, with stipules modifi ed in colleters in this separation had been maintained in the subsequent several species (Endress & Bruyns 2000; Capelli et al. -

Milkweeds for Monarch Butterflies
Milkweeds for Monarch Butterflies Milkweed (Asclepias spp.) are the required host plants for caterpillars of the monarch butterfly, playing a critical role in the monarch’s life cycle. The loss of milkweed plants in the monarch’s breeding areas and travel paths is believed to be a significant contribution to the decline of monarch populations across North America. Milkweed is a perennial plant. Add milkweed to your butterfly garden. Asclepias species produce some of the most complex flowers in the plant kingdom. Five petals reflex backwards revealing a gynostegium (fused stamen filaments and styles) surrounded by a five-member corona in a paired hood and horn structure with the hood acting as a sheath for the inner horn. Glands holding pollinia are found between the hoods. The size, shape and color of the horns and hoods are often important identifying characteristics for species in the genus Asclepias. Leaf shapes and spacing also help in identifying specific milkweeds. Texas has several varieties: 1. Antelope Horn - Asclepias asperula. Has fuzzy leaves and an odd greenish-white bloom. Is a clump-forming, 1-2 ft. tall, with an upright or sprawling habit. Stems are densely covered with minute hairs. Leaves are 4-8 inches long, narrow, irregularly grouped, thick and often folded length- wise. As the green seed follicles grow in length and begin to curve, they resemble antelope horns with curving and pointed shape of the seedpods. Prefers to grow in well drained soil in full sun. Appropriate for wildscapes Asclepias asperula and large plantings. High cardenolides. Antelope horn 2. Zizotes - Asclepias oenotheroides. -

Green Antelopehorn Milkweed Tolerance to Right-Of-Way Herbicides
Green Antelopehorn Milkweed Tolerance to Right-of-Way Herbicides Milkweed has a dual history: it has been explored as a potential crop, while others saw it as a weed. Some of the earliest European settlers to North America, colonists in Pomeioc, Virginia, used milkweed seedpod floss to make undergarments (Gerarde, 1597). Henkel (1907) stated American Indians used butterfly (Asclepias tuberosa), common (Asclepias syriaca), and swamp (Asclepias incarnata) milkweed medicinally. Whiting (1943) summarized published literature on cultivation of various species of milkweed for rubber, oil, or fiber and cited work by Neish and Burns (1921) suggesting the floss could be used to fill life preservers. Seedpods were collected, Figure 1. Green antelopehorn milkweed in flower. dried, and processed for that purpose during World War II. More recently, entrepreneurs habitat on privately owned property, federally managed marketed pillows, comforters, and clothing filled with floss lands, and electric, utility, and highway rights-of-way. as a down substitute (Knudson and Sayler, 1992). The USDA Plants Database (2018) lists 20 species In contrast, Darlington (1847) listed butterfly milkweed of milkweed native to Mississippi. In a recent survey of as the primary weedy species of Asclepias in cultivated Mississippi highway right-of-way vegetation, three species fields. As a physician, he also stated the medicinal value of milkweed were found on roadsides maintained by the of milkweed was insignificant. Fifty years later, common Mississippi Department of Transportation. In 800 plots milkweed was listed as one of 200 agricultural weeds in randomly scattered across Mississippi, green antelopehorn the USDA Yearbook of Agriculture 1895 (USDA, 1896). (Asclepias viridis) (Figure 1) was found in four plots, Georgia (1927) described control methods for four species common milkweed was found in two plots, and Michaux’s of milkweed in his text and reported the value of butterfly, milkweed (Asclepias michauxii) was found in one plot (V.