Insights Into Thymus Development and Viral Thymic Infections
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Horse Gamma Globulin Stabilized in 0.3 Molar Glycine to a Ph of Approximately 6.8
LPD Reference: ATG-SIN-0414/1 Date of Last Revision: 08 September 2014 Country: Singapore Reference document: CDS Version 3.0, effective date: 17-Mar-2014 Reason for change: LPD update in accordance with CDS 3.0 ; 2014-09-08 IR: 1. Remove entire proposed subsection <Renal Transplant Prophylaxis> under ‘Pharmacodynamic Properties - Clinical Studies’ & 2. Relocate paragraph “Clinical trials […] standard supportive care alone” from <Therapeutic Indications> to < Pharmacodynamic Properties - Clinical Studies>, subsection <Aplastic Anemia>. Atgam® lymphocyte immune globulin, anti-thymocyte globulin [equine] sterile solution For Intravenous Use only DESCRIPTION ATGAM Sterile Solution contains lymphocyte immune globulin, anti-thymocyte globulin [equine]. It is the purified, concentrated, and sterile gamma globulin, primarily monomeric IgG, from hyperimmune serum of horses immunized with human thymus lymphocytes. Anti-thymocyte globulin (equine) is a transparent to slightly opalescent aqueous protein solution. It may appear colorless to faintly pink or brown and is nearly odorless. It may develop a slight granular or flaky deposit during storage. (For information about in-line filters, see Infusion Instructions in the POSOLOGY AND METHOD OF ADMINISTRATION.) Before release for clinical use, each lot of anti-thymocyte globulin (equine) is tested to assure its ability to inhibit rosette formation between human peripheral lymphocytes and sheep red blood cells in vitro. In each lot, antibody activity against human red blood cells and platelets is also measured and determined to be within acceptable limits. Only lots that test negative for antihuman serum protein antibody, antiglomerular basement membrane antibody and pyrogens are released. Each milliliter of anti-thymocyte globulin (equine) contains 50 mg of horse gamma globulin stabilized in 0.3 molar glycine to a pH of approximately 6.8. -

MCB 50 Immunity and Disease Lecture Outline MHC Restriction Jan
MCB 50 Immunity and Disease Lecture Outline MHC Restriction Jan. 31, 2001 I. Definition of self MHC restriction; MHC restriction is the requirement that APC or target cells express MHC molecules that the T cell recognizes as self in order for T cell to respond to the antigen presented by that APC or target cell. (T cells will only recognize antigens presented by their own MHC molecules.) CD8 T cells bind class I MHC which are expressed on most cells in the body. CD4 T cells bind class II MHC which are only expressed on specialized APCs. APCs primarily reside in 2 ° lymphoid tissue (lymph nodes, spleen) but can be found in most tissues. II. MHC Genetics Mouse MHC gene complex called H-2. Human MHC gene complex called HLA. MHC Molecules Mouse H-2 Human HLA Class I K,D,L A, B, C Class II I-A, I-E DR, DQ, DP Definitions: Alleles functional forms of the same genes. Heterozygous different allele at each locus. Homozygous same allele at each locus. Haplotype is the total set of MHC alleles present on one chromosome. For each MHC gene locus you have 2 copies which can be denoted by their allele classification. Most humans will be heterozygous and will have different alleles for each of the different HLA loci. (e.g. A2/A12, B17/B83, C3/C37, DR3/DR4 etc). Syngeneic identical at all MHC loci same allele on both chromosomes. (inbred mice or identical twins). Allogeneic --genetically different at MHC different alleles. Every person gets a chromosome from each biological parent which contains an MHC molecule at each locus. -

Reactive Stroma in Human Prostate Cancer: Induction of Myofibroblast Phenotype and Extracellular Matrix Remodeling1
2912 Vol. 8, 2912–2923, September 2002 Clinical Cancer Research Reactive Stroma in Human Prostate Cancer: Induction of Myofibroblast Phenotype and Extracellular Matrix Remodeling1 Jennifer A. Tuxhorn, Gustavo E. Ayala, Conclusions: The stromal microenvironment in human Megan J. Smith, Vincent C. Smith, prostate cancer is altered compared with normal stroma and Truong D. Dang, and David R. Rowley2 exhibits features of a wound repair stroma. Reactive stroma is composed of myofibroblasts and fibroblasts stimulated to Departments of Molecular and Cellular Biology [J. A. T., T. D. D., express extracellular matrix components. Reactive stroma D. R. R.] and Pathology [G. E. A., M. J. S., V. C. S.] Baylor College of Medicine, Houston, Texas 77030 appears to be initiated during PIN and evolve with cancer progression to effectively displace the normal fibromuscular stroma. These studies and others suggest that TGF-1isa ABSTRACT candidate regulator of reactive stroma during prostate can- Purpose: Generation of a reactive stroma environment cer progression. occurs in many human cancers and is likely to promote tumorigenesis. However, reactive stroma in human prostate INTRODUCTION cancer has not been defined. We examined stromal cell Activation of the host stromal microenvironment is pre- phenotype and expression of extracellular matrix compo- dicted to be a critical step in adenocarcinoma growth and nents in an effort to define the reactive stroma environ- progression (1–5). Several human cancers have been shown to ment and to determine its ontogeny during prostate cancer induce a stromal reaction or desmoplasia as a component of progression. carcinoma progression. However, the specific mechanisms of Experimental Design: Normal prostate, prostatic intra- stromal cell activation are not known, and the extent to which epithelial neoplasia (PIN), and prostate cancer were exam- stroma regulates the biology of tumorigenesis is not fully un- ined by immunohistochemistry. -

1-Phosphate-Lyase-Deficient Mice Development in Sphingosine
Discontinued Postnatal Thymocyte Development in Sphingosine 1-Phosphate-Lyase-Deficient Mice This information is current as Claudia Weber, Andreas Krueger, Anika Münk, Constantin of October 1, 2021. Bode, Paul P. Van Veldhoven and Markus H. Gräler J Immunol 2009; 183:4292-4301; Prepublished online 11 September 2009; doi: 10.4049/jimmunol.0901724 http://www.jimmunol.org/content/183/7/4292 Downloaded from Supplementary http://www.jimmunol.org/content/suppl/2009/09/10/jimmunol.090172 Material 4.DC1 http://www.jimmunol.org/ References This article cites 48 articles, 20 of which you can access for free at: http://www.jimmunol.org/content/183/7/4292.full#ref-list-1 Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists by guest on October 1, 2021 • Fast Publication! 4 weeks from acceptance to publication *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2009 by The American Association of Immunologists, Inc. All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. The Journal of Immunology Discontinued Postnatal Thymocyte Development in Sphingosine 1-Phosphate-Lyase-Deficient Mice1,2 Claudia Weber,3* Andreas Krueger,3* Anika Mu¨nk,* Constantin Bode,* Paul P. -

Repertoire Selection from Data on the Mature T Cell Deriving Quantitative
Deriving Quantitative Constraints on T Cell Selection from Data on the Mature T Cell Repertoire This information is current as Vincent Detours, Ramit Mehr and Alan S. Perelson of October 2, 2021. J Immunol 2000; 164:121-128; ; doi: 10.4049/jimmunol.164.1.121 http://www.jimmunol.org/content/164/1/121 Downloaded from References This article cites 75 articles, 24 of which you can access for free at: http://www.jimmunol.org/content/164/1/121.full#ref-list-1 Why The JI? Submit online. http://www.jimmunol.org/ • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication *average by guest on October 2, 2021 Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2000 by The American Association of Immunologists All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. Deriving Quantitative Constraints on T Cell Selection from Data on the Mature T Cell Repertoire1 Vincent Detours,*†‡ Ramit Mehr,§ and Alan S. Perelson2* The T cell repertoire is shaped in the thymus through positive and negative selection. Thus, data about the mature repertoire may be used to infer information on how TCR generation and selection operate. -

Profiling Microdissected Epithelium and Stroma to Model Genomic Signatures for Cervical Carcinogenesis Accommodating for Covariates
Research Article Profiling Microdissected Epithelium and Stroma to Model Genomic Signatures for Cervical Carcinogenesis Accommodating for Covariates David Gius,1 Margo C. Funk,2 Eric Y. Chuang,1 Sheng Feng,3 Phyllis C. Huettner,4 Loan Nguyen,2 C. Matthew Bradbury,1 Mark Mishra,1 Shuping Gao,1 Barbara M. Buttin,2 David E. Cohn,2 Matthew A. Powell,2 Neil S. Horowitz,2 Bradford P. Whitcomb,2 and JanetS. Rader 2 1Radiation Oncology Branch, Center for Cancer Research, National Cancer Institute, NIH, Bethesda, Maryland and 2Division of Gynecologic Oncology, Department of Obstetrics and Gynecology; 3Division of Biostatistics; and 4Lauren V. Ackerman Laboratory of Surgical Pathology, Washington University School of Medicine, St. Louis, Missouri Abstract (CIN 1–3) and finally to squamous cell carcinoma antigen (SCCA) This study is the first comprehensive, integrated approach to have been well characterized. Histologically, CIN 1 consists of examine grade-specific changes in gene expression along the immature basal-type cells involving the lower third of the entire neoplastic spectrum of cervical intraepithelial neoplasia epithelium. In CIN 2, these immature basal-type cells involve more (CIN) in the process of cervical carcinogenesis. This was than the lower third, whereas CIN 3 involves the full thickness of the accomplished by identifying gene expression signatures of epithelium. In addition, higher CIN grades exhibit nuclear crowding, disease progression using cDNA microarrays to analyze RNA pleomorphism, loss of cell polarity, and increased mitotic activity from laser-captured microdissected epithelium and underlying (1). These transitions seemto be well conserved and, as such, stroma from normal cervix, graded CINs, cancer, and patient- provide an intriguing systemto use genomicsto identify the early matched normal cervical tissues. -

Thymic Nurse Cells Participate in Heterotypic
Send Orders for Reprints to [email protected] 828 Current Molecular Medicine 2015, 15, 828-835 Thymic Nurse Cells Participate in Heterotypic Internalization and Repertoire Selection of Immature Thymocytes; Their Removal from the Thymus of Autoimmune Animals May be Important to Disease Etiology J.C. Guyden1, M. Martinez2, R.V.E. Chilukuri1, V. Reid3, F. Kelly4 and M.-O.D. Samms*,1 1Department of Biology, The City College of New York, New York, NY 10031, USA 2Department of Biology College of Arts and Sciences, Tuskegee University, Armstrong Hall, Room 107, 1200 West Montgomery Road, Tuskegee, AL 36088, USA 3The Hall Perrine Cancer Center, Department of Surgery, Mercy Medical Center Cedar Rapids, IA, Division of Surgical Oncology & Endocrine Surgery, The University of Iowa Hospitals & Clinics, 200 Hawkins Dr, Iowa City, IA 52242, USA 4Essential Health, St Mary’s Medical Center, 407 East 3rd Street, Duluth, MN 55805, USA M.-O.D. Samms Abstract: Thymic nurse cells (TNCs) are specialized epithelial cells that reside in the thymic cortex. The initial report of their discovery in 1980 showed TNCs to contain up to 200 thymocytes within specialized vacuoles in their cytoplasm. Much has been reported since that time to determine the function of this heterotypic internalization event that exists between TNCs and developing thymocytes. In this review, we discuss the literature reported that describes the internalization event and the role TNCs play during T cell development in the thymus as well as why these multicellular complexes may be important in inhibiting the development of autoimmune diseases. Keywords: Thymic nurse cells, internalization, MHC restriction, lupus erythromatosus. -

Colposcopy of the Uterine Cervix
THE CERVIX: Colposcopy of the Uterine Cervix • I. Introduction • V. Invasive Cancer of the Cervix • II. Anatomy of the Uterine Cervix • VI. Colposcopy • III. Histology of the Normal Cervix • VII: Cervical Cancer Screening and Colposcopy During Pregnancy • IV. Premalignant Lesions of the Cervix The material that follows was developed by the 2002-04 ASCCP Section on the Cervix for use by physicians and healthcare providers. Special thanks to Section members: Edward J. Mayeaux, Jr, MD, Co-Chair Claudia Werner, MD, Co-Chair Raheela Ashfaq, MD Deborah Bartholomew, MD Lisa Flowers, MD Francisco Garcia, MD, MPH Luis Padilla, MD Diane Solomon, MD Dennis O'Connor, MD Please use this material freely. This material is an educational resource and as such does not define a standard of care, nor is intended to dictate an exclusive course of treatment or procedure to be followed. It presents methods and techniques of clinical practice that are acceptable and used by recognized authorities, for consideration by licensed physicians and healthcare providers to incorporate into their practice. Variations of practice, taking into account the needs of the individual patient, resources, and limitation unique to the institution or type of practice, may be appropriate. I. AN INTRODUCTION TO THE NORMAL CERVIX, NEOPLASIA, AND COLPOSCOPY The uterine cervix presents a unique opportunity to clinicians in that it is physically and visually accessible for evaluation. It demonstrates a well-described spectrum of histological and colposcopic findings from health to premalignancy to invasive cancer. Since nearly all cervical neoplasia occurs in the presence of human papillomavirus infection, the cervix provides the best-defined model of virus-mediated carcinogenesis in humans to date. -

Nomina Histologica Veterinaria, First Edition
NOMINA HISTOLOGICA VETERINARIA Submitted by the International Committee on Veterinary Histological Nomenclature (ICVHN) to the World Association of Veterinary Anatomists Published on the website of the World Association of Veterinary Anatomists www.wava-amav.org 2017 CONTENTS Introduction i Principles of term construction in N.H.V. iii Cytologia – Cytology 1 Textus epithelialis – Epithelial tissue 10 Textus connectivus – Connective tissue 13 Sanguis et Lympha – Blood and Lymph 17 Textus muscularis – Muscle tissue 19 Textus nervosus – Nerve tissue 20 Splanchnologia – Viscera 23 Systema digestorium – Digestive system 24 Systema respiratorium – Respiratory system 32 Systema urinarium – Urinary system 35 Organa genitalia masculina – Male genital system 38 Organa genitalia feminina – Female genital system 42 Systema endocrinum – Endocrine system 45 Systema cardiovasculare et lymphaticum [Angiologia] – Cardiovascular and lymphatic system 47 Systema nervosum – Nervous system 52 Receptores sensorii et Organa sensuum – Sensory receptors and Sense organs 58 Integumentum – Integument 64 INTRODUCTION The preparations leading to the publication of the present first edition of the Nomina Histologica Veterinaria has a long history spanning more than 50 years. Under the auspices of the World Association of Veterinary Anatomists (W.A.V.A.), the International Committee on Veterinary Anatomical Nomenclature (I.C.V.A.N.) appointed in Giessen, 1965, a Subcommittee on Histology and Embryology which started a working relation with the Subcommittee on Histology of the former International Anatomical Nomenclature Committee. In Mexico City, 1971, this Subcommittee presented a document entitled Nomina Histologica Veterinaria: A Working Draft as a basis for the continued work of the newly-appointed Subcommittee on Histological Nomenclature. This resulted in the editing of the Nomina Histologica Veterinaria: A Working Draft II (Toulouse, 1974), followed by preparations for publication of a Nomina Histologica Veterinaria. -

Tumor-Associated Stromal Cells As Key Contributors to the Tumor Microenvironment Karen M
Bussard et al. Breast Cancer Research (2016) 18:84 DOI 10.1186/s13058-016-0740-2 REVIEW Open Access Tumor-associated stromal cells as key contributors to the tumor microenvironment Karen M. Bussard1,2, Lysette Mutkus3, Kristina Stumpf3, Candelaria Gomez-Manzano4 and Frank C. Marini1,3* Abstract The tumor microenvironment is a heterogeneous population of cells consisting of the tumor bulk plus supporting cells. It is becoming increasingly evident that these supporting cells are recruited by cancer cells from nearby endogenous host stroma and promote events such as tumor angiogenesis, proliferation, invasion, and metastasis, as well as mediate mechanisms of therapeutic resistance. In addition, recruited stromal cells range in type and include vascular endothelial cells, pericytes, adipocytes, fibroblasts, and bone-marrow mesenchymal stromal cells. During normal wound healing and inflammatory processes, local stromal cells change their phenotype to become that of reactive stroma. Under certain conditions, however, tumor cells can co-opt these reactive stromal cells and further transition them into tumor-associated stromal cells (TASCs). These TASCs express higher levels of proteins, including alpha-smooth muscle actin, fibroblast activating protein, and matrix metalloproteinases, compared with their normal, non-reactive counterparts. TASCs are also known to secrete many pro-tumorigenic factors, including IL-6, IL-8, stromal-derived factor-1 alpha, vascular endothelial growth factor, tenascin-C, and matrix metalloproteinases, among others, which recruit additional tumor and pro-tumorigenic cells to the developing microenvironment. Here, we review the current literature pertaining to the origins of recruited host stroma, contributions toward tumor progression, tumor-associated stromal cells, and mechanisms of crosstalk between endogenous host stroma and tumor cells. -
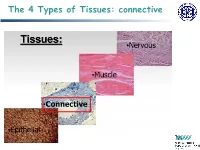
The 4 Types of Tissues: Connective
The 4 Types of Tissues: connective Connective Tissue General structure of CT cells are dispersed in a matrix matrix = a large amount of extracellular material produced by the CT cells and plays a major role in the functioning matrix component = ground substance often crisscrossed by protein fibers ground substance usually fluid, but it can also be mineralized and solid (bones) CTs = vast variety of forms, but typically 3 characteristic components: cells, large amounts of amorphous ground substance, and protein fibers. Connective Tissue GROUND SUBSTANCE In connective tissue, the ground substance is an amorphous gel-like substance surrounding the cells. In a tissue, cells are surrounded and supported by an extracellular matrix. Ground substance traditionally does not include fibers (collagen and elastic fibers), but does include all the other components of the extracellular matrix . The components of the ground substance vary depending on the tissue. Ground substance is primarily composed of water, glycosaminoglycans (most notably hyaluronan ), proteoglycans, and glycoproteins. Usually it is not visible on slides, because it is lost during the preparation process. Connective Tissue Functions of Connective Tissues Support and connect other tissues Protection (fibrous capsules and bones that protect delicate organs and, of course, the skeletal system). Transport of fluid, nutrients, waste, and chemical messengers is ensured by specialized fluid connective tissues, such as blood and lymph. Adipose cells store surplus energy in the form of fat and contribute to the thermal insulation of the body. Embryonic Connective Tissue All connective tissues derive from the mesodermal layer of the embryo . The first connective tissue to develop in the embryo is mesenchyme , the stem cell line from which all connective tissues are later derived. -

HLA-Restricted, Processing- and Metabolism-Independent Pathway of Drug Recognition by Human Alpha Beta T Lymphocytes
HLA-restricted, processing- and metabolism-independent pathway of drug recognition by human alpha beta T lymphocytes. M P Zanni, … , S Valitutti, W J Pichler J Clin Invest. 1998;102(8):1591-1598. https://doi.org/10.1172/JCI3544. Research Article T cell recognition of drugs is explained by the hapten-carrier model, implying covalent binding of chemically reactive drugs to carrier proteins. However, most drugs are nonreactive and their recognition by T cells is unclear. We generated T cell clones from allergic individuals specific to sulfamethoxazole, lidocaine (nonreactive drugs), and cef-triaxone (per se reactive beta-lactam antibiotic) and compared the increase of intracellular free calcium concentration ([Ca2+]i) and the kinetics of T cell receptor (TCR) downregulation of these clones by drug-specific stimulations. All drugs tested induced an MHC-restricted, dose- and antigen-presenting cell (APC)-dependent TCR downregulation on specific CD4(+) and CD8(+) T cell clones. Chemically nonreactive drugs elicited an immediate and sustained [Ca2+]i increase and a rapid TCR downregulation, but only when these drugs were added in solution to APC and clone. In contrast, the chemically reactive hapten ceftriaxone added in solution needed > 6 h to induce TCR downregulation. When APC were preincubated with ceftriaxone, a rapid downregulation of the TCR and cytokine secretion was observed, suggesting a stable presentation of a covalently modified peptide. Our data demonstrate two distinct pathways of drug presentation to activated specific T cells. The per se reactive ceftriaxone is presented after covalent binding to carrier peptides. Nonreactive drugs can be recognized by specific alphabeta+ T cells via a nonconventional presentation pathway based on a labile binding […] Find the latest version: https://jci.me/3544/pdf HLA-restricted, Processing- and Metabolism-independent Pathway of Drug Recognition by Human ab T Lymphocytes Martin P.