ENVIRONMENTAL CHEMISTRY 1: Atmosphere
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CARI-7 Documentation: Radiation Transport in the Atmosphere
DOT/FAA/AM-21/5 Office of Aerospace Medicine Washington, DC 20591 CARI Documentation: Radiation Transport in the Atmosphere Kyle Copeland Civil Aerospace Medical Institute Federal Aviation Administration Oklahoma City, OK 73125Location/Address March 2021 Final Report NOTICE This document is disseminated under the sponsorship of the U.S. Department of Transportation in the interest of information exchange. The United States Government assumes no liability for the contents thereof. _________________ This publication and all Office of Aerospace Medicine technical reports are available in full-text from the Civil Aerospace Medical Institute’s publications Web site: (www.faa.gov/go/oamtechreports) Technical Report Documentation Page 1. Report No. 2. Government Accession No. 3. Recipient's Catalog No. DOT/FAA/AM-21/5 4. Title and Subtitle 5. Report Date CARI-7 DOCUMENTATION: RADIATION TRANSPORT IN March 2021 THE ATMOSPHERE 6. Performing Organization Code 7. Author(s) 8. Performing Organization Report No. Copeland, K. 9. Performing Organization Name and Address 10. Work Unit No. (TRAIS) Civil Aerospace Medical Institute FAA 11. Contract or Grant No. 12. Sponsoring Agency name and Address 13. Type of Report and Period Covered Office of Aerospace Medicine Federal Aviation Administration 800 Independence Ave., S.W. Washington, DC 20591 14. Sponsoring Agency Code 15. Supplemental Notes 16. Abstract Primary cosmic radiation from both the Sun and interstellar space enters Earth's atmosphere in varying amounts. Outside of Earth's atmosphere, cosmic radiation is modulated by solar activity and Earth's magnetic field. Once the radiation enters Earth's atmosphere, it interacts with Earth's atmosphere in the same manner regardless of its point of origin (solar or galactic). -

Hohonu Volume 5 (PDF)
HOHONU 2007 VOLUME 5 A JOURNAL OF ACADEMIC WRITING This publication is available in alternate format upon request. TheUniversity of Hawai‘i is an Equal Opportunity Affirmative Action Institution. VOLUME 5 Hohonu 2 0 0 7 Academic Journal University of Hawai‘i at Hilo • Hawai‘i Community College Hohonu is publication funded by University of Hawai‘i at Hilo and Hawai‘i Community College student fees. All production and printing costs are administered by: University of Hawai‘i at Hilo/Hawai‘i Community College Board of Student Publications 200 W. Kawili Street Hilo, Hawai‘i 96720-4091 Phone: (808) 933-8823 Web: www.uhh.hawaii.edu/campuscenter/bosp All rights revert to the witers upon publication. All requests for reproduction and other propositions should be directed to writers. ii d d d d d d d d d d d d d d d d d d d d d d Table of Contents 1............................ A Fish in the Hand is Worth Two on the Net: Don’t Make me Think…different, by Piper Seldon 4..............................................................................................Abortion: Murder-Or Removal of Tissue?, by Dane Inouye 9...............................An Etymology of Four English Words, with Reference to both Grimm’s Law and Verner’s Law by Piper Seldon 11................................Artifacts and Native Burial Rights: Where do We Draw the Line?, by Jacqueline Van Blarcon 14..........................................................................................Ayahuasca: Earth’s Wisdom Revealed, by Jennifer Francisco 16......................................Beak of the Fish: What Cichlid Flocks Reveal About Speciation Processes, by Holly Jessop 26................................................................................. Climatic Effects of the 1815 Eruption of Tambora, by Jacob Smith 33...........................Columnar Joints: An Examination of Features, Formation and Cooling Models, by Mary Mathis 36.................... -

Summary of a Program Review Held at Huntsville, Alabama October 19-21, 1982
Summary of a program review held at Huntsville, Alabama October 19-21, 1982 - TECH LIBRARY KAFEI, NM lllllllsllllllRlRllffllilrml OOSSE!?b NASA Conference Publication 2259 NASA/MSFCFY-82 Atmospheric Processes Research Review Compiled by Robert E. Turner George C. Marshall Space Flight Center Marshall Space Flight Center, Alabama Summary of a program review held at Huntsville, Alabama October 19-21, 1982 National Aeronautics and Space Administration Sclontlflc and Tochnlcal InformatIon Branch 1983 ACKNOWLEDGMENTS The productive inputs and comments from the participants and attendees in the Atmospheric Processes Research Review contributed very much to the success of the review. The opportunity provided for everyone to become better acquainted with the work of other investigators and to see how the research relates to the overall objective of NASA's Atmospheric Processes Research Program was an important aspect of the review. Appreciation is expressed to all those who participated in the review. The organizers trust that participation will provide each with a better frame of reference from which to proceed with the next year's research activities. ii PREFACE Each year NASA supports research in various disciplinary program areas. The coordination and exchange of information among those sponsored by NASA to conduct research studies are important elements of each program. The Office of Space Science and Applications and the Office of Aeronautics and Space Technology, via Announcements of Opportunity (AO), Application Notices (AN),etc., invites interested investigators throughout the country to communicate their research ideas within NASA and in institutions. The proposals in the Atmospheric Processes Research area selected and assigned to the NASA Marshall Space Flight Center's (MSFC's) Atmospheric Sciences Division for technical monitorship, together with the research efforts included in the FY-82 MSFC Research and Technology Operating Plan (RTOP1 I are the source of principal focus for the NASA/MSFC FY-82 Atmospheric Processes Research Review. -

The Stratopause Evolution During Different Types of Sudden Stratospheric Warming Event
Clim Dyn DOI 10.1007/s00382-014-2292-4 The stratopause evolution during different types of sudden stratospheric warming event Etienne Vignon · Daniel M. Mitchell Received: 18 February 2014 / Accepted: 5 August 2014 © The Author(s) 2014. This article is published with open access at Springerlink.com Abstract Recent work has shown that the vertical struc- Keywords Stratopause · Sudden stratospheric warming · ture of the Arctic polar vortex during different types of MERRA data · Polar vortex · sudden stratospheric warming (SSW) events can be very Middle atmospheric circulation distinctive. Specifically, SSWs can be classified into polar vortex displacement events or polar vortex splitting events. This paper aims to study the Arctic stratosphere during 1 Introduction such events, with a focus on the stratopause using the Mod- ern Era-Restrospective analysis for Research and Applica- The stratopause is characterised by a reversal of the atmos- tions reanalysis data set. The reanalysis dataset is compared pheric lapse rate at around 50 km (~1 hPa). While strato- against two independent satellite reconstructions for valida- spheric ozone heating is responsible for the stratopause pres- tion purposes. During vortex displacement events, the strat- ence at sunlit latitudes, westward gravity wave drag (and to opause temperature and pressure exhibit a wave-1 structure a lesser extent, stationary gravity wave drag) maintains the and are in quadrature whereas during vortex splitting events stratopause in the polar night jet (Hitchman et al. 1989). they exhibit a wave-2 structure. For both types of SSW the Indeed, the westward and stationary gravity wave (GW) temperature anomalies at the stratopause are shown to be breaking induces a mesospheric meridional flow toward the generated by ageostrophic vertical motions. -

Earth's Atmospheric Layers
Earth's atmospheric layers Earth's atmospheric layers Lesson plan (Polish) Lesson plan (English) Earth's atmospheric layers Source: licencja: CC 0, [online], dostępny w internecie: www.pixabay.pl. Link to the lesson Before you start you should know what the place of the atmosphere is in relation to the lithosphere, hydrosphere, biosphere and pedosphere; that the Earth's atmosphere is the part of the Earth and moves with it. You will learn explain the term „atmosphere”; name gases that form the air and their percentage share; name permanent and variable components of atmospheric air; name the layers of the atmosphere; discuss the role of the ozone layer; characterize the effects of the ozone hole and the greenhouse effect. Nagranie dostępne na portalu epodreczniki.pl nagranie abstraktu What layers is the atmosphere built of? In the Earth's atmosphere we distinguish 5 main layers characterized by specific features and 4 intermediate layers called pauses. The boundaries between them are conventional and change depending on the geographical latitude, terrain and season of the year. The closest one to the surface of the earth is the troposphere. Its thickness ranges from 7 km (in winter) to 10 km (in summer) above the poles, and 15‐18 km above the equator. The main feature that allows determining the boundary of the troposphere is the drop in the air temperature with an increase of about 0,6°C per 100 m. In the upper layer of the troposphere, the temperature reaches -55°C (above arctic regions) to -70°C (above equatorial regions). Above this layer there is a thin tropopause with the constant temperature, and above it there is the stratosphere extending up to a height of about 50 km, in which the air temperature rises to reach 0°C. -

Elemental Geosystems, 5E (Christopherson) Chapter 2 Solar Energy, Seasons, and the Atmosphere
Elemental Geosystems, 5e (Christopherson) Chapter 2 Solar Energy, Seasons, and the Atmosphere 1) Our planet and our lives are powered by A) energy derived from inside Earth. B) radiant energy from the Sun. C) utilities and oil companies. D) shorter wavelengths of gamma rays, X-rays, and ultraviolet. Answer: B 2) Which of the following is true? A) The Sun is the largest star in the Milky Way Galaxy. B) The Milky Way is part of our Solar System. C) The Sun produces energy through fusion processes. D) The Sun is also a planet. Answer: C 3) Which of the following is true about the Milky Way galaxy in which we live? A) It is a spiral-shaped galaxy. B) It is one of millions of galaxies in the universe. C) It contains approximately 400 billion stars. D) All of the above are true. E) Only A and B are true. Answer: D 4) The planetesimal hypothesis pertains to the formation of the A) universe. B) galaxy. C) planets. D) ocean basins. Answer: C 5) The flattened structure of the Milky Way is revealed by A) the constellations of the Zodiac. B) a narrow band of hazy light that stretches across the night sky. C) the alignment of the planets in the solar system. D) the plane of the ecliptic. Answer: B 6) Earth and the Sun formed specifically from A) the galaxy. B) unknown origins. C) a nebula of dust and gases. D) other planets. Answer: C 7) Which of the following is not true of stars? A) They form in great clouds of gas and dust known as nebula. -

Methods of Oabservation at Sea Meteorological Soundings in The
WORLD METEOROLOGICAL ORGANIZATION WORLD METEOROLOGICAL ORGANIZATION TECHNICAL NOTE No. 2 TECHNICAL NOTE No. 60 METHODS OF OABSERVATION AT SEA METEOROLOGICAL SOUNDINGS IN THE PARTUPPER I – SEA SURFACEATMOSPHERE TEMPERATURE by W.W. KELLOGG WMO-No.WMO-No. 153. 26. TP. 738 Secretariat of the World Meteorological Organization – Geneva – Switzerland THE WMO The WOTld :Meteol'ological Organization (Wl\IO) is a specialized agency of the United Nations of which 125 States and Territories arc Members. It was created: to facilitate international co~operation in the establishment of networks of stations and centres to provide meteorological services and observationsI to promote the establishment and maintenance of systems for the rapid exchange of meteorological information, to promote standardization of meteorological observations and ensure the uniform publication of observations and statistics. to further the application of rneteol'ology to Rviatioll, shipping, agricultul"C1 and other human activities. to encourage research and training in meteorology. The machinery of the Organization consists of: The World Nleteorological Congress, the supreme body of the o.rganization, brings together the delegates of all Members once every four years to determine general policies for the fulfilment of the purposes of the Organization, to adopt Technical Regulations relating to international meteorological practice and to determine the WMO programme, The Executive Committee is composed of 21 dil'cetors of national meteorological services and meets at least once a yeae to conduct the activities of the Organization and to implement the decisions taken by its Members in Congress, to study and make recommendations Oll matters affecting international meteorology and the opel'ation of meteorological services. -

Today: Upper Atmosphere/Ionosphere
Today: Upper Atmosphere/Ionosphere • Review atmospheric Layers – Follow the energy! – What heats the Stratosphere? • Mesosphere – Most turbulent layer – why? • Ionosphere – What is a Plasma? The Solar Spectrum: The amount of energy the Sun produces at a given wavelength is determined by its temperature. This is a general property of any Black Body such as a star or the heating element on the stove. • The Sun is about 5270 K and produces most light in the visible. • The atmosphere is transparent in the visible and so over half of the solar energy reaches the ground. • Some gasses absorb certain wavelengths of sunlight, and thus some energy is absorbed directly into the atmosphere. Depositing Energy Energy from the Sun may be transmitted directly to the ground, absorbed in the atmosphere, or reflected from clouds or the Sun back into space. Some re-radiated heat from the ground is absorbed by the atmosphere, further heating it. The Troposhphere The troposphere is the region of the atmosphere we live in. The primary source of energy in the troposphere is heat (infrared light ) radiated from the ground. This means that it is warmest at the bottom and coolest at the top. Temperature drops about 11.5 ° F for each km of altitude. When pressure and temperature (with temp. faster) drop with altitude it triggers convection . Convection makes the troposphere unstable, but in a good way. Dominant Region The Troposphere contains 80% of the mass in the atmosphere and 99% of the water in the atmosphere. Water Vapor, CO 2, methane, nitrous oxide, ozone, chloroflorocarbons are all greenhouse gases Convection (both horizontal and vertical) produces weather in the atmosphere. -

The Earth's Atmosphere
A Resource Booklet for SACE Stage 1 Earth and Environmental Science The following pages have been prepared by practicing teachers of SACE Earth and Environmental Science. The six Chapters are aligned with the six topics described in the SACE Stage 1 subject outline. They aim to provide an additional source of contexts and ideas to help teachers plan to teach this subject. For further information, including the general and assessment requirements of the course see: https://www.sace.sa.edu.au/web/earth-and-environmental- science/stage-1/planning-to-teach/subject-outline A Note for Teachers The resources in this booklet are not intended for ‘publication’. They are ‘drafts’ that have been developed by teachers for teachers. They can be freely used for educational purposes, including course design, topic and lesson planning. Each Chapter is a living document, intended for continuous improvement in the future. Teachers of Earth and Environmental Science are invited to provide feedback, particularly suggestions of new contexts, field-work and practical investigations that have been found to work well with students. Your suggestions for improvement would be greatly appreciated and should be directed to our project coordinator:: [email protected] Preparation of this booklet has been coordinated and funded by the Geoscience Pathways Project, under the sponsorship of the Geological Society of Australia (GSA) and the Teacher Earth Science Education Program (TESEP). In-kind support has been provided by the SA Department of Energy and Mining (DEM) and the Geological Survey of South Australia (GSSA). FAIR USE: The teachers named alongside each chapter of this document have researched available resources, selected and collated these notes and images from a wide range of sources. -
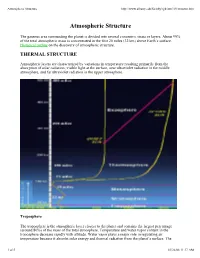
Atmospheric Structure
Atmospheric Structure http://www.albany.edu/faculty/rgk/atm101/structur.htm Atmospheric Structure The gaseous area surrounding the planet is divided into several concentric strata or layers. About 99% of the total atmospheric mass is concentrated in the first 20 miles (32 km) above Earth's surface. Historical outline on the discovery of atmospheric structure. THERMAL STRUCTURE Atmospheric layers are characterized by variations in temperature resulting primarily from the absorption of solar radiation; visible light at the surface, near ultraviolet radiation in the middle atmosphere, and far ultraviolet radiation in the upper atmosphere. Troposphere The troposphere is the atmospheric layer closest to the planet and contains the largest percentage (around 80%) of the mass of the total atmosphere. Temperature and water vapor content in the troposphere decrease rapidly with altitude. Water vapor plays a major role in regulating air temperature because it absorbs solar energy and thermal radiation from the planet's surface. The 1 of 5 01/26/08 11:17 AM Atmospheric Structure http://www.albany.edu/faculty/rgk/atm101/structur.htm troposphere contains 99 % of the water vapor in the atmosphere. Water vapor concentrations vary with latitude. They are greatest above the tropics, where they may be as high as 3 %, and decrease toward the polar regions. All weather phenomena occur within the troposphere, although turbulence may extend into the lower portion of the stratosphere. Troposphere means "region of mixing" and is so named because of vigorous convective air currents within the layer. The upper boundary of the layer, known as the tropopause, ranges in height from 5 miles (8 km) near the poles up to 11 miles (18 km) above the equator. -

The Earth's Atmosphere
TheThe EaEartrthh’s’s AtAtmomosphsphereere Earth’Earth’ss AtmospAtmospheherree AtmospAtmospherehere isis thethe gaseougaseouss layerlayer thatthat surrsurroundsounds thethe eeartharth IsIs aa mixmixtureture ofof ggasesases tthathat isis nanaturturallallyy odorodorlleess,ss, colourcolourless,less, tastetastelessless andand forformlessmless AiAirr isis blendblendeded soso thorthoroughlyoughly tthathat itit behavebehavess asas ifif itit wweerere aa singlesingle ggasas ThThee AtAtmospmospheherere AiAirr isis heldheld toto tthehe earearthth bbyy thethe forceforce ofof ggravity:ravity: TThehe furfurtherther awayaway frfromom thethe ssoourceurce ofof ggravitatravitatiiononalal attrattractionaction (e(eartharth)) ththee lowerlower thethe aattrattractictioonalnal forforcece MoMorere airair molecmoleculesules araree heldheld clclososerer toto thethe earearthth ththanan atat highehigherr elevaelevatitioonsns AtmosphAtmosphereere isis mormoree dedensense nenearar ththee sursurfaceface tthanhan atat highhigherer elevatioelevationsns ThThee AtAtmospmospheherere NoNo rerealal “t“top”op” –– atmatmospherospheree ddriftsrifts ofofff toto nothingnothingnessness aabovebove aboaboutut 101000 kmkm 97%97% ofof tthehe weighweightt andand 100%100% ofof tthehe watewaterr vapovaporr reresisiddee iinn thethe bottbottomom 3300 kmkm DDensiensittyy TThhee dendensisittyy (m(massass perper ununiitt volume)volume) ofof thethe atmoatmossppherehere quicklyquickly decrdecreaseseases wwithith iinncreacreassiningg elevatelevatiioonn aboaboveve seasea llevevelel DensityDensity -

Layers of the Atmosphere
Name ________________________ Layers of the Atmosphere By Jack Fearing, Lincoln Junior High School, Hibbing, Minnesota OBJECTIVE: To discover how the atmosphere can be divided into layers based on temperature changes at different heights, by making a graph. BACKGROUND: The atmosphere can be divided into four layers based on temperature variations. The layer closest to the Earth is called the troposphere. Above this layer is the stratosphere, followed by the mesosphere, then the thermosphere. The upper boundaries between these layers are known as the tropopause, the stratopause, and the mesopause, respectively. Temperature variations in the four layers are due to the way solar energy is absorbed as it moves downward through the atmosphere. The Earth’s surface is the primary absorber of solar energy. Some of this energy is reradiated by the Earth as heat, which warms the overlying troposphere. The global average temperature in the troposphere rapidly decreases with altitude until the tropopause, the boundary between the troposphere and the stratosphere. The temperature begins to increase with altitude in the stratosphere. This warming is caused by a form of oxygen called ozone (O3) absorbing ultraviolet radiation from the sun. Ozone protects us from most of the sun’s ultraviolet radiation, which can cause cancer, genetic mutations, and sunburn. Scientists are concerned that human activity is contributing to a decrease in stratospheric ozone. Nitric oxide, which is the exhaust of high-flying jets, and chlorofluorocarbons (CFCs), which are used as refrigerants, may contribute to ozone depletion. At the stratopause, the temperature stops increasing with altitude. The overlying mesosphere does not absorb solar radiation, so the temperature decreases with altitude.