The Earth's Atmosphere
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CARI-7 Documentation: Radiation Transport in the Atmosphere
DOT/FAA/AM-21/5 Office of Aerospace Medicine Washington, DC 20591 CARI Documentation: Radiation Transport in the Atmosphere Kyle Copeland Civil Aerospace Medical Institute Federal Aviation Administration Oklahoma City, OK 73125Location/Address March 2021 Final Report NOTICE This document is disseminated under the sponsorship of the U.S. Department of Transportation in the interest of information exchange. The United States Government assumes no liability for the contents thereof. _________________ This publication and all Office of Aerospace Medicine technical reports are available in full-text from the Civil Aerospace Medical Institute’s publications Web site: (www.faa.gov/go/oamtechreports) Technical Report Documentation Page 1. Report No. 2. Government Accession No. 3. Recipient's Catalog No. DOT/FAA/AM-21/5 4. Title and Subtitle 5. Report Date CARI-7 DOCUMENTATION: RADIATION TRANSPORT IN March 2021 THE ATMOSPHERE 6. Performing Organization Code 7. Author(s) 8. Performing Organization Report No. Copeland, K. 9. Performing Organization Name and Address 10. Work Unit No. (TRAIS) Civil Aerospace Medical Institute FAA 11. Contract or Grant No. 12. Sponsoring Agency name and Address 13. Type of Report and Period Covered Office of Aerospace Medicine Federal Aviation Administration 800 Independence Ave., S.W. Washington, DC 20591 14. Sponsoring Agency Code 15. Supplemental Notes 16. Abstract Primary cosmic radiation from both the Sun and interstellar space enters Earth's atmosphere in varying amounts. Outside of Earth's atmosphere, cosmic radiation is modulated by solar activity and Earth's magnetic field. Once the radiation enters Earth's atmosphere, it interacts with Earth's atmosphere in the same manner regardless of its point of origin (solar or galactic). -

Inert Gas & Winemaking a Moremanual !™ by Shea A.J
Inert Gas & Winemaking A MoreManual !™ by Shea A.J. Comfort www.MoreWineMaking.com 1–800–823–0010 The Importance of Inert Gas therefore eliminating any headspace (as is the case when filling/topping-up barrels), but as we shall see in the next During aging, if a wine is not protected from both microbial section this may not always be practical. spoilage and oxygen at all times it is likely to spoil. Protecting wine usually involves maintaining proper SO 2 Expansion & Contraction — The Need For levels and keeping containers full. Additionally, purging your headspaces with inert gas to effectively remove the Headspaces: oxygen greatly increases the amount of protection. When Unless you are in a situation with a guarantee of temperature stability, as with a glycol-jacketed tank, or a it comes to using SO2, the benefits are widely understood and in-depth information describing its usage is readily temperature-controlled storage area, tanks and carboys available in most winemaking literature. Yet, often when should have a small headspace kept at the top (note that these texts refer to purging with inert gas they fail to barrels should not have any space in them when filled/ explain the actual, step-by-step techniques needed to topped). This headspace is needed because it helps to do so. It is important be aware that creating an effective compensate for the expansion and contraction of the blanket of gas to protect your wine requires more than liquid due to ambient temperature changes (remember just shooting some Argon into the headspace of your things expand when heated and contract when cooled). -

Biological Effects of Noble Gases
Physiol. Res. 56 (Suppl. 1): S39-S44, 2007 Biological Effects of Noble Gases J. RŮŽIČKA, J. BENEŠ, L. BOLEK, V. MARKVARTOVÁ Department of Biophysics, Medical Faculty of Charles University, Plzeň, Czech Republic Received May 23, 2007 Accepted May 29, 2007 On-line available May 31, 2007 Summary Noble gases are known for their inertness. They do not react chemically with any element at normal temperature and pressure. Through that, some of them are known to be biologically active by their sedative, hypnotic and analgesic properties. Common inhalation anesthetics are characterized by some disadvantages (toxicity, decreased cardiac output, etc). Inhalation of xenon introduces anesthesia and has none of the above disadvantages, hence xenon seems to be the anesthetic gas of the future (with just one disadvantage – its cost). It is known that argon has similar anesthetic properties (under hyperbaric conditions), which is much cheaper and easily accessible. The question is if this could be used in clinical practice, in anesthesia of patients who undergo treatment in the hyperbaric chamber. Xenon was found to be organ-protective. Recent animal experiments indicated that xenon decreases infarction size after ischemic attack on brain or heart. The goal of our study is to check if hyperbaric argon has properties similar to those of xenon. Key words Noble gases • Xenon• Argon • Diving • Anesthesia • Stroke Introduction it is the point of this work. Above all, available information and our own observation concerning xenon Helium, neon, argon, krypton, xenon and radon and argon will be gathered here. are elements of the eighth group of the periodic table of Argon is the longest known and the least rare gas elements. -

CHEMICAL ACTIVITY of NOBLE GASES Kr and Xe and ITS IMPACT on FISSION GAS ACCUMULATION in the IRRADIATED UO2 FUEL M
ANNUAL REPORT 2005 Nuclear Technology in Energy Generation CHEMICAL ACTIVITY OF NOBLE GASES Kr AND Xe AND ITS IMPACT ON FISSION GAS ACCUMULATION IN THE IRRADIATED UO2 FUEL M. Szuta Institute of Atomic Energy It is generally accepted that most of the insoluble We can further assume that above a limiting value inert gas atoms Xe and Kr produced during fissioning of fission fluency (burn-up) a more intensive process of are retained in the fuel irradiated at a temperature lower irradiation induced chemical interaction occurs. Signifi- than the threshold. Some authors assume random diffu- cant part of fission gas product is thus expected to be sion of gas atoms to grain boundaries and consider the chemically bound in the matrix of UO2. effect of trapping the atoms at inter-granular bubbles From the moment of discovering the rare gases until saturation occurs. Others confirmed that bubbles (helium, neon, argon, krypton, xenon and radon) at the tend to concentrate in the grain boundaries during irra- end of XIX century until to the beginning of sixties diation. Likewise, some authors further assume that years of XX century it was considered that the noble most of the gas atoms are retained in solution in the gases are chemically inactive. matrix of grains being there immobilised or are precipi- The nobility of rare gases started to deteriorate af- tated into small fission gas bubbles. ter the first xenon compound was found by Barlett in The experimental data presented in the open litera- 1962 [1]. Barlett showed that the noble gases are capa- ture imply that we can assume that after irradiation ble of forming what one could consider as normal exposure in excess of 1018 fissions/cm3 the single gas chemical compounds, compelling chemists to readjust atom diffusion can be disregarded in description of considerably their thinking regarding these elements. -

Elemental Geosystems, 5E (Christopherson) Chapter 2 Solar Energy, Seasons, and the Atmosphere
Elemental Geosystems, 5e (Christopherson) Chapter 2 Solar Energy, Seasons, and the Atmosphere 1) Our planet and our lives are powered by A) energy derived from inside Earth. B) radiant energy from the Sun. C) utilities and oil companies. D) shorter wavelengths of gamma rays, X-rays, and ultraviolet. Answer: B 2) Which of the following is true? A) The Sun is the largest star in the Milky Way Galaxy. B) The Milky Way is part of our Solar System. C) The Sun produces energy through fusion processes. D) The Sun is also a planet. Answer: C 3) Which of the following is true about the Milky Way galaxy in which we live? A) It is a spiral-shaped galaxy. B) It is one of millions of galaxies in the universe. C) It contains approximately 400 billion stars. D) All of the above are true. E) Only A and B are true. Answer: D 4) The planetesimal hypothesis pertains to the formation of the A) universe. B) galaxy. C) planets. D) ocean basins. Answer: C 5) The flattened structure of the Milky Way is revealed by A) the constellations of the Zodiac. B) a narrow band of hazy light that stretches across the night sky. C) the alignment of the planets in the solar system. D) the plane of the ecliptic. Answer: B 6) Earth and the Sun formed specifically from A) the galaxy. B) unknown origins. C) a nebula of dust and gases. D) other planets. Answer: C 7) Which of the following is not true of stars? A) They form in great clouds of gas and dust known as nebula. -

Today: Upper Atmosphere/Ionosphere
Today: Upper Atmosphere/Ionosphere • Review atmospheric Layers – Follow the energy! – What heats the Stratosphere? • Mesosphere – Most turbulent layer – why? • Ionosphere – What is a Plasma? The Solar Spectrum: The amount of energy the Sun produces at a given wavelength is determined by its temperature. This is a general property of any Black Body such as a star or the heating element on the stove. • The Sun is about 5270 K and produces most light in the visible. • The atmosphere is transparent in the visible and so over half of the solar energy reaches the ground. • Some gasses absorb certain wavelengths of sunlight, and thus some energy is absorbed directly into the atmosphere. Depositing Energy Energy from the Sun may be transmitted directly to the ground, absorbed in the atmosphere, or reflected from clouds or the Sun back into space. Some re-radiated heat from the ground is absorbed by the atmosphere, further heating it. The Troposhphere The troposphere is the region of the atmosphere we live in. The primary source of energy in the troposphere is heat (infrared light ) radiated from the ground. This means that it is warmest at the bottom and coolest at the top. Temperature drops about 11.5 ° F for each km of altitude. When pressure and temperature (with temp. faster) drop with altitude it triggers convection . Convection makes the troposphere unstable, but in a good way. Dominant Region The Troposphere contains 80% of the mass in the atmosphere and 99% of the water in the atmosphere. Water Vapor, CO 2, methane, nitrous oxide, ozone, chloroflorocarbons are all greenhouse gases Convection (both horizontal and vertical) produces weather in the atmosphere. -

Fire Code Section 5309 Inert Gas Systems Used in Commercial
Section 5309 Inert Gas Systems Used in Commercial, Manufacturing or Industrial Applications is added as follows: SECTION 5309 INERT GAS SYSTEMS USED IN COMMERCIAL, MANUFACTURING OR INDUSTRIAL APPLICATIONS 5309.1 General. Inert gas systems with more than 100 pounds (45.4 kg) of an inert gas or any system using any amount of an inert gas below grade used in a commercial, manufacturing or industrial application, such as water treatment with pH balancing, food processing or laboratories shall comply with Sections 5309.1 through 5309.8. Inert gases include but are not limited to argon, helium, nitrogen and carbon dioxide. Provisions of Section 5307 are applicable where CO2 is used. Exceptions: 1. Medical gas systems 2. Gaseous Fire suppression systems 3. Carbon dioxide gas enrichment systems in accordance with Section 5310 5309.2 Permits. Permits shall be required in accordance with Sections 105 and in accordance with Denver Fire Department policy. 5309.3 Equipment. The storage, use, and handling of inert gases shall be in accordance with IFC Chapters 53 and 55, as amended, and the applicable requirements of NFPA 55. All equipment utilized in compressed gas systems shall be compatible with the intended gas and use. 5309.3.1 Containers, cylinders and tanks. Gas storage containers, cylinders and tanks shall be designed, fabricated, tested and labeled with manufactures’ specifications and shall be maintained in accordance with the regulations of DOTn 49 CFR, Parts 100-185 or the ASME Boiler and Pressure Vessel Code, Section VIII. 5309.3.1.1 Location. Location of gas storage containers, cylinders and tanks, inside or outside the building, shall be at an approved location. -

The Noble Gases
INTERCHAPTER K The Noble Gases When an electric discharge is passed through a noble gas, light is emitted as electronically excited noble-gas atoms decay to lower energy levels. The tubes contain helium, neon, argon, krypton, and xenon. University Science Books, ©2011. All rights reserved. www.uscibooks.com Title General Chemistry - 4th ed Author McQuarrie/Gallogy Artist George Kelvin Figure # fig. K2 (965) Date 09/02/09 Check if revision Approved K. THE NOBLE GASES K1 2 0 Nitrogen and He Air P Mg(ClO ) NaOH 4 4 2 noble gases 4.002602 1s2 O removal H O removal CO removal 10 0 2 2 2 Ne Figure K.1 A schematic illustration of the removal of O2(g), H2O(g), and CO2(g) from air. First the oxygen is removed by allowing the air to pass over phosphorus, P (s) + 5 O (g) → P O (s). 20.1797 4 2 4 10 2s22p6 The residual air is passed through anhydrous magnesium perchlorate to remove the water vapor, Mg(ClO ) (s) + 6 H O(g) → Mg(ClO ) ∙6 H O(s), and then through sodium hydroxide to remove 18 0 4 2 2 4 2 2 the carbon dioxide, NaOH(s) + CO2(g) → NaHCO3(s). The gas that remains is primarily nitrogen Ar with about 1% noble gases. 39.948 3s23p6 36 0 The Group 18 elements—helium, K-1. The Noble Gases Were Kr neon, argon, krypton, xenon, and Not Discovered until 1893 83.798 radon—are called the noble gases 2 6 4s 4p and are noteworthy for their rela- In 1893, the English physicist Lord Rayleigh noticed 54 0 tive lack of chemical reactivity. -

Argon: Systematic Review on Neuro- and Organoprotective Properties of an “Inert” Gas
Int. J. Mol. Sci. 2014, 15, 18175-18196; doi:10.3390/ijms151018175 OPEN ACCESS International Journal of Molecular Sciences ISSN 1422-0067 www.mdpi.com/journal/ijms Review Argon: Systematic Review on Neuro- and Organoprotective Properties of an “Inert” Gas Anke Höllig 1,2, Anita Schug 1, Astrid V. Fahlenkamp 2, Rolf Rossaint 2, Mark Coburn 2,* and Argon Organo-Protective Network (AON) † 1 Department of Neurosurgery, University RWTH Aachen, 52074 Aachen, Germany; E-Mails: [email protected] (A.H.); [email protected] (A.S.) 2 Department of Anesthesiology, University RWTH Aachen, 52074 Aachen, Germany; E-Mails: [email protected] (A.V.F.); [email protected] (R.R.) † Members are listed in Appendix. * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +49-241-80-88179; Fax: +49-241-80-82406. External Editor: Katalin Prokai-Tatrai Received: 14 August 2014; in revised form: 12 September 2014 / Accepted: 23 September 2014 / Published: 10 October 2014 Abstract: Argon belongs to the group of noble gases, which are regarded as chemically inert. Astonishingly some of these gases exert biological properties and during the last decades more and more reports demonstrated neuroprotective and organoprotective effects. Recent studies predominately use in vivo or in vitro models for ischemic pathologies to investigate the effect of argon treatment. Promising data has been published concerning pathologies like cerebral ischemia, traumatic brain injury and hypoxic ischemic encephalopathy. However, models applied and administration of the therapeutic gas vary. Here we provide a systematic review to summarize the available data on argon’s neuro- and organoprotective effects and discuss its possible mechanism of action. -

Viability of Inert Matrix Fuel in Reducing Plutonium Amounts in Reactors
IAEA-TECDOC-1516 Viability of inert matrix fuel in reducing plutonium amounts in reactors August 2006 IAEA-TECDOC-1516 Viability of inert matrix fuel in reducing plutonium amounts in reactors August 2006 The originating Section of this publication in the IAEA was: Nuclear Fuel Cycle and Materials Section International Atomic Energy Agency Wagramer Strasse 5 P.O. Box 100 A-1400 Vienna, Austria VIABILITY OF INERT MATRIX FUEL IN REDUCING PLUTONIUM AMOUNTS IN REACTORS IAEA, VIENNA, 2006 IAEA-TECDOC-1516 ISBN 92–0–110506–1 ISSN 1011–4289 © IAEA, 2006 Printed by the IAEA in Austria August 2006 FOREWORD Reactors around the world have produced more than 2000 tonnes of plutonium, contained in spent fuel, as separated forms through reprocessing, or as weapons-grade material. The recycling of plutonium as uranium–plutonium mixed oxide fuel derives additional energy from this resource; however, it does not speedily reduce growing plutonium inventories. The use of inert matrix fuel (IMF) in the current generation of reactors would provide a means of reducing plutonium inventories. The reduction of the accumulated plutonium by the use of IMF is a subject of great interest in several Member States. Work on IMF to date has been investigating the feasibility and reactor strategies for utilizing these fuels. Another important application of IMF is the reduction of minor actinide content, with or without plutonium. IMF can be used both to manage plutonium inventories and to address the long term radiotoxicity of spent fuel by minor actinide reduction in today’s reactors. IMF materials are also being considered for generation-IV reactors. -

The Earth's Atmosphere
A Resource Booklet for SACE Stage 1 Earth and Environmental Science The following pages have been prepared by practicing teachers of SACE Earth and Environmental Science. The six Chapters are aligned with the six topics described in the SACE Stage 1 subject outline. They aim to provide an additional source of contexts and ideas to help teachers plan to teach this subject. For further information, including the general and assessment requirements of the course see: https://www.sace.sa.edu.au/web/earth-and-environmental- science/stage-1/planning-to-teach/subject-outline A Note for Teachers The resources in this booklet are not intended for ‘publication’. They are ‘drafts’ that have been developed by teachers for teachers. They can be freely used for educational purposes, including course design, topic and lesson planning. Each Chapter is a living document, intended for continuous improvement in the future. Teachers of Earth and Environmental Science are invited to provide feedback, particularly suggestions of new contexts, field-work and practical investigations that have been found to work well with students. Your suggestions for improvement would be greatly appreciated and should be directed to our project coordinator:: [email protected] Preparation of this booklet has been coordinated and funded by the Geoscience Pathways Project, under the sponsorship of the Geological Society of Australia (GSA) and the Teacher Earth Science Education Program (TESEP). In-kind support has been provided by the SA Department of Energy and Mining (DEM) and the Geological Survey of South Australia (GSSA). FAIR USE: The teachers named alongside each chapter of this document have researched available resources, selected and collated these notes and images from a wide range of sources. -
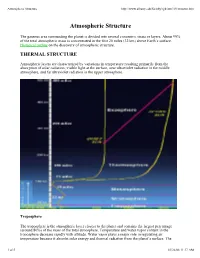
Atmospheric Structure
Atmospheric Structure http://www.albany.edu/faculty/rgk/atm101/structur.htm Atmospheric Structure The gaseous area surrounding the planet is divided into several concentric strata or layers. About 99% of the total atmospheric mass is concentrated in the first 20 miles (32 km) above Earth's surface. Historical outline on the discovery of atmospheric structure. THERMAL STRUCTURE Atmospheric layers are characterized by variations in temperature resulting primarily from the absorption of solar radiation; visible light at the surface, near ultraviolet radiation in the middle atmosphere, and far ultraviolet radiation in the upper atmosphere. Troposphere The troposphere is the atmospheric layer closest to the planet and contains the largest percentage (around 80%) of the mass of the total atmosphere. Temperature and water vapor content in the troposphere decrease rapidly with altitude. Water vapor plays a major role in regulating air temperature because it absorbs solar energy and thermal radiation from the planet's surface. The 1 of 5 01/26/08 11:17 AM Atmospheric Structure http://www.albany.edu/faculty/rgk/atm101/structur.htm troposphere contains 99 % of the water vapor in the atmosphere. Water vapor concentrations vary with latitude. They are greatest above the tropics, where they may be as high as 3 %, and decrease toward the polar regions. All weather phenomena occur within the troposphere, although turbulence may extend into the lower portion of the stratosphere. Troposphere means "region of mixing" and is so named because of vigorous convective air currents within the layer. The upper boundary of the layer, known as the tropopause, ranges in height from 5 miles (8 km) near the poles up to 11 miles (18 km) above the equator.