1. Atmospheric Basics
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CARI-7 Documentation: Radiation Transport in the Atmosphere
DOT/FAA/AM-21/5 Office of Aerospace Medicine Washington, DC 20591 CARI Documentation: Radiation Transport in the Atmosphere Kyle Copeland Civil Aerospace Medical Institute Federal Aviation Administration Oklahoma City, OK 73125Location/Address March 2021 Final Report NOTICE This document is disseminated under the sponsorship of the U.S. Department of Transportation in the interest of information exchange. The United States Government assumes no liability for the contents thereof. _________________ This publication and all Office of Aerospace Medicine technical reports are available in full-text from the Civil Aerospace Medical Institute’s publications Web site: (www.faa.gov/go/oamtechreports) Technical Report Documentation Page 1. Report No. 2. Government Accession No. 3. Recipient's Catalog No. DOT/FAA/AM-21/5 4. Title and Subtitle 5. Report Date CARI-7 DOCUMENTATION: RADIATION TRANSPORT IN March 2021 THE ATMOSPHERE 6. Performing Organization Code 7. Author(s) 8. Performing Organization Report No. Copeland, K. 9. Performing Organization Name and Address 10. Work Unit No. (TRAIS) Civil Aerospace Medical Institute FAA 11. Contract or Grant No. 12. Sponsoring Agency name and Address 13. Type of Report and Period Covered Office of Aerospace Medicine Federal Aviation Administration 800 Independence Ave., S.W. Washington, DC 20591 14. Sponsoring Agency Code 15. Supplemental Notes 16. Abstract Primary cosmic radiation from both the Sun and interstellar space enters Earth's atmosphere in varying amounts. Outside of Earth's atmosphere, cosmic radiation is modulated by solar activity and Earth's magnetic field. Once the radiation enters Earth's atmosphere, it interacts with Earth's atmosphere in the same manner regardless of its point of origin (solar or galactic). -

GEF2200 Spring 2018: Solutions Thermodynam- Ics 1
GEF2200 spring 2018: Solutions thermodynam- ics 1 A.1.T What is the difference between R and R∗? R∗ is the universal gas constant, with value 8.3143JK−1mol−1. R is the gas constant for a specific gas, given by R∗ R = (1) M where M is the molecular weight of the gas (usually given in g/mol). In other words, R takes into account the weight of the gas in question so that mass can be used in the equation of state. For the equation of state: m pV = nR∗T = R∗T = mRT (2) M It is important to note that we usually use mass m in units of [kg], which requires that the units of R is changed accordingly (if given in [J/gK], it must be multiplied by 1000 [g/kg]). A.2.T What is apparent molecular weight, and why do we use it? Apparent molecular weight is the average molecular weight for a mixture of gases. We introduce ∗ it to calculate a gas constant R = R =Md for the mixture , where Md is the apparent molecular weight of i different gases given by Equation (3.10): P m P m P n M M = i = i = i i d P mi n n Mi X ni = M (3) n i In meteorology the most common apparent molecular weight is the one of air. WH06 3.19 Determine the apparent molecular weight of the Venusian atmosphere, assuming that it consists of 95% CO2 and 5% N2 by volume. What is the gas constant for 1 kg of such an atmosphere? (Atomic weights of C, O and N are 12, 16 and 14 respectively.) Concentrations by volume (See exercise A.8.T): V v = N2 (4) N2 V V v = CO2 (5) CO2 V 1 Assuming ideal gas, we have total volume V = VN2 + VCO2 at a given temperature (T ) and pressure (p). -

Elemental Geosystems, 5E (Christopherson) Chapter 2 Solar Energy, Seasons, and the Atmosphere
Elemental Geosystems, 5e (Christopherson) Chapter 2 Solar Energy, Seasons, and the Atmosphere 1) Our planet and our lives are powered by A) energy derived from inside Earth. B) radiant energy from the Sun. C) utilities and oil companies. D) shorter wavelengths of gamma rays, X-rays, and ultraviolet. Answer: B 2) Which of the following is true? A) The Sun is the largest star in the Milky Way Galaxy. B) The Milky Way is part of our Solar System. C) The Sun produces energy through fusion processes. D) The Sun is also a planet. Answer: C 3) Which of the following is true about the Milky Way galaxy in which we live? A) It is a spiral-shaped galaxy. B) It is one of millions of galaxies in the universe. C) It contains approximately 400 billion stars. D) All of the above are true. E) Only A and B are true. Answer: D 4) The planetesimal hypothesis pertains to the formation of the A) universe. B) galaxy. C) planets. D) ocean basins. Answer: C 5) The flattened structure of the Milky Way is revealed by A) the constellations of the Zodiac. B) a narrow band of hazy light that stretches across the night sky. C) the alignment of the planets in the solar system. D) the plane of the ecliptic. Answer: B 6) Earth and the Sun formed specifically from A) the galaxy. B) unknown origins. C) a nebula of dust and gases. D) other planets. Answer: C 7) Which of the following is not true of stars? A) They form in great clouds of gas and dust known as nebula. -

Thickness and Thermal Wind
ESCI 241 – Meteorology Lesson 12 – Geopotential, Thickness, and Thermal Wind Dr. DeCaria GEOPOTENTIAL l The acceleration due to gravity is not constant. It varies from place to place, with the largest variation due to latitude. o What we call gravity is actually the combination of the gravitational acceleration and the centrifugal acceleration due to the rotation of the Earth. o Gravity at the North Pole is approximately 9.83 m/s2, while at the Equator it is about 9.78 m/s2. l Though small, the variation in gravity must be accounted for. We do this via the concept of geopotential. l A surface of constant geopotential represents a surface along which all objects of the same mass have the same potential energy (the potential energy is just mF). l If gravity were constant, a geopotential surface would also have the same altitude everywhere. Since gravity is not constant, a geopotential surface will have varying altitude. l Geopotential is defined as z F º ò gdz, (1) 0 or in differential form as dF = gdz. (2) l Geopotential height is defined as F 1 z Z º = ò gdz (3) g0 g0 0 2 where g0 is a constant called standard gravity, and has a value of 9.80665 m/s . l If the change in gravity with height is ignored, geopotential height and geometric height are related via g Z = z. (4) g 0 o If the local gravity is stronger than standard gravity, then Z > z. o If the local gravity is weaker than standard gravity, then Z < z. -

Vertical Structure
ESCI 341 – Atmospheric Thermodynamics Lesson 7 – Vertical Structure References: An Introduction to Dynamic Meteorology, Holton Introduction to Theoretical Meteorology, Hess Synoptic-dynamic Meteorology in Midlatitudes, Vol. 1, Bluestein ‘An example of uncertainty in sea level pressure reduction’, P.M. Pauley, Mon. Wea. Rev., 13, 1998, pp. 833-850 GEOPOTENTIAL l The acceleration due to gravity is not constant. It varies from place to place, with the largest variation due to latitude. o What we call gravity is the combination of the gravitational acceleration and the centrifugal acceleration from the Earth’s rotation. o Gravity at the North Pole is approximately 9.83 m/s2, while at the Equator it is about 9.78 m/s2. l Though small, the variation in gravity must be accounted for. We do this via the concept of geopotential. l Geopotential is essentially the potential energy per unit mass. l A surface of constant geopotential represents a surface along which all objects of the same mass have the same potential energy. l If gravity were constant, a geopotential surface would lie at a constant altitude. Since gravity is not constant, a geopotential surface will have varying altitude. l Geopotential is defined as z F º ò gdz, (1) 0 or in differential form as dF = gdz. l Geopotential height is defined as F 1 Z Zº=ò gdZ (2) gg000 2 where g0 is a constant called standard gravity, and has a value of 9.80665 m/s . o Geopotential height is expressed in geopotential meters, abbreviated as gpm. l If the change in gravity with height is ignored, geopotential height and geometric height are related via g Z = z. -

Today: Upper Atmosphere/Ionosphere
Today: Upper Atmosphere/Ionosphere • Review atmospheric Layers – Follow the energy! – What heats the Stratosphere? • Mesosphere – Most turbulent layer – why? • Ionosphere – What is a Plasma? The Solar Spectrum: The amount of energy the Sun produces at a given wavelength is determined by its temperature. This is a general property of any Black Body such as a star or the heating element on the stove. • The Sun is about 5270 K and produces most light in the visible. • The atmosphere is transparent in the visible and so over half of the solar energy reaches the ground. • Some gasses absorb certain wavelengths of sunlight, and thus some energy is absorbed directly into the atmosphere. Depositing Energy Energy from the Sun may be transmitted directly to the ground, absorbed in the atmosphere, or reflected from clouds or the Sun back into space. Some re-radiated heat from the ground is absorbed by the atmosphere, further heating it. The Troposhphere The troposphere is the region of the atmosphere we live in. The primary source of energy in the troposphere is heat (infrared light ) radiated from the ground. This means that it is warmest at the bottom and coolest at the top. Temperature drops about 11.5 ° F for each km of altitude. When pressure and temperature (with temp. faster) drop with altitude it triggers convection . Convection makes the troposphere unstable, but in a good way. Dominant Region The Troposphere contains 80% of the mass in the atmosphere and 99% of the water in the atmosphere. Water Vapor, CO 2, methane, nitrous oxide, ozone, chloroflorocarbons are all greenhouse gases Convection (both horizontal and vertical) produces weather in the atmosphere. -

The Earth's Atmosphere
A Resource Booklet for SACE Stage 1 Earth and Environmental Science The following pages have been prepared by practicing teachers of SACE Earth and Environmental Science. The six Chapters are aligned with the six topics described in the SACE Stage 1 subject outline. They aim to provide an additional source of contexts and ideas to help teachers plan to teach this subject. For further information, including the general and assessment requirements of the course see: https://www.sace.sa.edu.au/web/earth-and-environmental- science/stage-1/planning-to-teach/subject-outline A Note for Teachers The resources in this booklet are not intended for ‘publication’. They are ‘drafts’ that have been developed by teachers for teachers. They can be freely used for educational purposes, including course design, topic and lesson planning. Each Chapter is a living document, intended for continuous improvement in the future. Teachers of Earth and Environmental Science are invited to provide feedback, particularly suggestions of new contexts, field-work and practical investigations that have been found to work well with students. Your suggestions for improvement would be greatly appreciated and should be directed to our project coordinator:: [email protected] Preparation of this booklet has been coordinated and funded by the Geoscience Pathways Project, under the sponsorship of the Geological Society of Australia (GSA) and the Teacher Earth Science Education Program (TESEP). In-kind support has been provided by the SA Department of Energy and Mining (DEM) and the Geological Survey of South Australia (GSSA). FAIR USE: The teachers named alongside each chapter of this document have researched available resources, selected and collated these notes and images from a wide range of sources. -
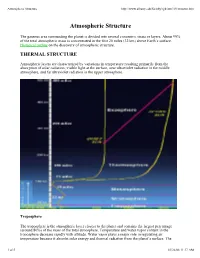
Atmospheric Structure
Atmospheric Structure http://www.albany.edu/faculty/rgk/atm101/structur.htm Atmospheric Structure The gaseous area surrounding the planet is divided into several concentric strata or layers. About 99% of the total atmospheric mass is concentrated in the first 20 miles (32 km) above Earth's surface. Historical outline on the discovery of atmospheric structure. THERMAL STRUCTURE Atmospheric layers are characterized by variations in temperature resulting primarily from the absorption of solar radiation; visible light at the surface, near ultraviolet radiation in the middle atmosphere, and far ultraviolet radiation in the upper atmosphere. Troposphere The troposphere is the atmospheric layer closest to the planet and contains the largest percentage (around 80%) of the mass of the total atmosphere. Temperature and water vapor content in the troposphere decrease rapidly with altitude. Water vapor plays a major role in regulating air temperature because it absorbs solar energy and thermal radiation from the planet's surface. The 1 of 5 01/26/08 11:17 AM Atmospheric Structure http://www.albany.edu/faculty/rgk/atm101/structur.htm troposphere contains 99 % of the water vapor in the atmosphere. Water vapor concentrations vary with latitude. They are greatest above the tropics, where they may be as high as 3 %, and decrease toward the polar regions. All weather phenomena occur within the troposphere, although turbulence may extend into the lower portion of the stratosphere. Troposphere means "region of mixing" and is so named because of vigorous convective air currents within the layer. The upper boundary of the layer, known as the tropopause, ranges in height from 5 miles (8 km) near the poles up to 11 miles (18 km) above the equator. -

The Earth's Atmosphere
TheThe EaEartrthh’s’s AtAtmomosphsphereere Earth’Earth’ss AtmospAtmospheherree AtmospAtmospherehere isis thethe gaseougaseouss layerlayer thatthat surrsurroundsounds thethe eeartharth IsIs aa mixmixtureture ofof ggasesases tthathat isis nanaturturallallyy odorodorlleess,ss, colourcolourless,less, tastetastelessless andand forformlessmless AiAirr isis blendblendeded soso thorthoroughlyoughly tthathat itit behavebehavess asas ifif itit wweerere aa singlesingle ggasas ThThee AtAtmospmospheherere AiAirr isis heldheld toto tthehe earearthth bbyy thethe forceforce ofof ggravity:ravity: TThehe furfurtherther awayaway frfromom thethe ssoourceurce ofof ggravitatravitatiiononalal attrattractionaction (e(eartharth)) ththee lowerlower thethe aattrattractictioonalnal forforcece MoMorere airair molecmoleculesules araree heldheld clclososerer toto thethe earearthth ththanan atat highehigherr elevaelevatitioonsns AtmosphAtmosphereere isis mormoree dedensense nenearar ththee sursurfaceface tthanhan atat highhigherer elevatioelevationsns ThThee AtAtmospmospheherere NoNo rerealal “t“top”op” –– atmatmospherospheree ddriftsrifts ofofff toto nothingnothingnessness aabovebove aboaboutut 101000 kmkm 97%97% ofof tthehe weighweightt andand 100%100% ofof tthehe watewaterr vapovaporr reresisiddee iinn thethe bottbottomom 3300 kmkm DDensiensittyy TThhee dendensisittyy (m(massass perper ununiitt volume)volume) ofof thethe atmoatmossppherehere quicklyquickly decrdecreaseseases wwithith iinncreacreassiningg elevatelevatiioonn aboaboveve seasea llevevelel DensityDensity -

Virtual Temperature Thickness Sea Level Pressure Potential Temperature Equivalent Potential Temperature
Important Quantities that we will use often in class to examine Atmospheric Processes: Virtual Temperature Thickness Sea Level Pressure Potential Temperature Equivalent Potential Temperature (See P. 57-64 and 195-217 of Bluestein, Vol. I for a detailed discussion) Review of Basic Thermodynamic Equations Ideal gas law relates pressure, density, and temperature for an ideal gas (air is P = pressure considered an ideal gas) ρ = air density R = gas constant P = !RT T = Temperature "P Z = height Hydrostatic equation: = #g$ "z • Upward-directed pressure gradient force per unit mass is balanced by the downward directed force of gravity per unit mass. • Pressure at a given height is given by the weight of the column above per unit horizontal area. ! • Pressure decreases with height, the extent to which an air parcel is compressed also decreases with height. • When applied, you are making the hydrostatic approximation. When is this approximation not valid? • Using these equations, many useful quantities and relations can be derived. Virtual Temperature: The temperature that a parcel of dry air would have if it were at the same pressure and had the same density as moist air. P = pressure = dry air density Derivation: ρd ρv = vapor density Start with ideal gas law for moist air: ρ = air density R= gas constant Rv = vapor gas constant P = !RT (461.5 J kg-1 °-1) Rd = dry air gas constant (287 J kg-1 °-1) P = !d RdT + !v RvT T = Temperature (K) Now treat moist air as if it were dry by introducing the virtual temperature Tv P = (!d Rd + !v Rv )T = (!d -

Reduction of Atmospheric Pressure (Preliminary Report on Problems Involved)
WJVlO ~, -- ,1. WORLD METEOROLOGICAL ORGANIZATION \ P TECHNICAL NOTE N° 7 REDUCTION OF ATMOSPHERIC PRESSURE (PRELIMINARY REPORT ON PROBLEMS INVOLVED) PRICE: Sw. fr. 3.- I WMO· N° 36. TP. 12 1 Secretariat of the World Meteorolqgical Organization • Geneva • Switzerland 1954. III REDUCTION DE LA PRESS ION ATMOSPHERIQUE (Rapport preliminaire sur les problemes souleves par cette question) La premiere partie de cette Note technique contient un resume des metho des utilisees par 59 Services meteorologiques pour reduire la valeur de la pres sion observee par les stations meteorologiques a la valeur qui aurait ete cons tatee si la station avait ete situee au niveau moyen de la mer. Les meteorolo gistes se sont penches depuis les premiers jours de la science meteorologique sur ce probleme fort controverse. Les informations figurant dans la premlere partie ont ete obtenues a la suite d'une enquete faite par le Secretariat de l'OMM en 1952/53; elles demon trent la necessite de proceder a une normalisation plus poussee des methodes de reduction de la pression qui sont appliquees dans les diverses regions du monde. La seconde partie de la Note contient un rapport prepare au caurs de la premlere session de la Commission des Instruments et des Methodes d'Observation (Toronto, 1953) par M. L.P. Harrison, President du Groupe de travail de Barome trie. Ce rapport examine l'ensemble du probleme de la reduction de la pression en commen~ant par l'analyse des rapports soumis a ce sujet a la session de To ronto en 1953 _et par un examen critique des methodes de reduction de la pres sion employees actuellement par les Services meteorologiques. -

The Problem of Using Mariner Iv Ionospheric Densities to Deduce a Model of the Martian Atmospheric Structure
i- . 8 -’ I 8 SPACE RESEARCH COORDI - ----’ --“T=D 1 GPO PRICE $- $- CFSTI PRICE(S) $ / Microfiche (MF) , Ls ff 853 July 65 THE PROBLEM OF USING MARINER IV IONOSPHERIC DENSITIES TO DEDUCE A MODEL OF THE MARTIAN ATMOSPHERIC STRUCTURE BY T. M. DONAHUE DEPARTMENT OF PHYSICS SRCC REPORT NO. 41 UNIVERSITY OF PITTSBURGH PITTSBURGH, PENNSYLVANIA 7 APRIL 1967 THE PROBLEM OF USING MARINER IV IONOSPHERIC DENSITIES TO DEDUCE A MODEL OF THE MARTIAN ATMOSPHERIC STRUCTURE T. M. Donahue University of Pittsburgh Pittsburgh, Pennsylvania April 1967 Reproduction in whole or in part is permissible for any purpose of the United States Government. The Problem of Using Mariner IV Ionospheric Densities to Deduce a Model of the Martian Atmospheric Structure (Presented at the Conference on The Atmospheres of Mars and Venus Kitt Peak National Iaboratory, February, 1967) T. M. Donahue University of Pittsburgh ABSTRACT An F1 layer maximum would apparently occur fifteen kilometers too high in the Martian ionosphere even though the atmosphere were pure C02 in a model with high temperature mesopause and thermopause. It is pointed out, however, that a similar calculation of electron densities in the earth's ionosphere starting with a reasonable model for neutral structure and temperature would also disagree with the observed profile of electron density in the F1 region. It is argued that where no ionospheric model based on unforced assumptions manifestly supports any atmospheric model it is unreasonable to rely on ionospheric properties to discriminate between models. Recently I suggested(') that the Martian ionosphere observed by Mariner IV(2) might be an F1 layer if the temperature profile was close to that calcuhted by Chamberlain and M~Elroy(~)and the lower atmosphere was almost pure C02 with a smface pressure of 6 mb.