Advantage Value for Diabetes Eligible Drug List for the State of Minnesota
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Treatment Patterns, Persistence and Adherence Rates in Patients with Type 2 Diabetes Mellitus in Japan: a Claims- Based Cohort Study
Open access Research BMJ Open: first published as 10.1136/bmjopen-2018-025806 on 1 March 2019. Downloaded from Treatment patterns, persistence and adherence rates in patients with type 2 diabetes mellitus in Japan: a claims- based cohort study Rimei Nishimura,1 Haruka Kato,2 Koichi Kisanuki,2 Akinori Oh,2 Shinzo Hiroi,2 Yoshie Onishi,3 Florent Guelfucci,4 Yukio Shimasaki2 To cite: Nishimura R, Kato H, ABSTRACT Strengths and limitations of this study Kisanuki K, et al. Treatment Objective To determine real-world trends in antidiabetic patterns, persistence and drug use, and persistence and adherence, in Japanese ► This retrospective evaluation of administrative adherence rates in patients patients with type 2 diabetes mellitus (T2DM). with type 2 diabetes mellitus claims data (2011–2015) using the Japan Medical Design Retrospective evaluation of administrative claims in Japan: a claims-based Data Center (JMDC) and Medical Data Vision (MDV) data (2011–2015) using the Japan Medical Data Center cohort study. BMJ Open databases was conducted to determine real-world (JMDC) and Medical Data Vision (MDV) databases. 2019;9:e025806. doi:10.1136/ trends in antidiabetic drug use, and persistence and Setting Analysis of two administrative claims databases bmjopen-2018-025806 adherence, in Japanese patients with type 2 dia- for Japanese patients with T2DM. betes mellitus (T2DM); 40 908 and 90 421 patients ► Prepublication history and Participants Adults (aged ≥18 years) with an International additional material for this were included from the JMDC and MDV databases, Classification of Diseases, 10th Revision code of T2DM and paper are available online. To respectively. at least one antidiabetic drug prescription. -

Effect of Oral Hypoglycaemic Agents on Glucose Tolerance in Pancreatic Diabetes
Gut: first published as 10.1136/gut.13.4.285 on 1 April 1972. Downloaded from Gut, 1972, 13, 285-288 Effect of oral hypoglycaemic agents on glucose tolerance in pancreatic diabetes B. I. JOFFE, W. P. U. JACKSON, S. BANK, AND A. I. VINIK From the Department of Medicine, Witwatersrand University Medical School, Johannesburg, the Gastro- intestinal and Endocrine Research Units of Cape Town University Medical School, and the Chemical Pathology Department of Natal University, South Africa SUMMARY The short-term therapeutic effect of oral hypoglycaemic agents has been assessed in 12 patients with symptomatic diabetes secondary to chronic pancreatitis (pancreatic diabetes). In six patients who had moderate to severe carbohydrate intolerance, associated with severe insulino- paenia during arginine infusion, the potent sulphonylurea chlorpropamide produced no change in the fasting blood glucose level after two weeks of treatment. This contrasted with the significant reduction produced in a matched group of maturity-onset primary diabetics. The six patients with milder diabetes, and a greater (although still subnormal) insulin secretory capacity, showed an improvement in oral glucose tolerance during the first hour following glucose administration while on chlorpropamide. When the biguanide phenformin was substituted for chlorpropamide in five of these patients, a statistically insignificant improvement in glucose tolerance was observed during treatment. Applications of these findings to the practical management of pancreatic diabetes are briefly http://gut.bmj.com/ considered. Chronic pancreatitis is frequently complicated by and two women, ranging from 30 to 67 years of age. diabetes (pancreatic diabetes). Recent studies The diagnosis of pancreatitis was confirmed on the utilizing immunoassay procedures (Joffe, Bank, basis of a gross abnormality in at least two aspects of Jackson, Keller, O'Reilly, and Vinik, 1968; Anderson the pancreatic function test, namely, a low volume of on September 24, 2021 by guest. -

Dipeptidyl Peptidase-4 Inhibitors and Combinations
Dipeptidyl Peptidase-4 Inhibitors & Combinations Policy Number: C5169A CRITERIA EFFECTIVE DATES: ORIGINAL EFFECTIVE DATE LAST REVIEWED DATE NEXT REVIEW DATE 06/2016 10/30/2019 10/30/2020 J CODE TYPE OF CRITERIA LAST P&T APPROVAL/VERSION NA RxPA Q4 2019 20191030C5169-A PRODUCTS AFFECTED: KAZANO (alogliptin/metformin), KOMBIGLYZE XR (saxagliptin/metformin extended-release), NESINA (alogliptin), ONGLYZA (saxagliptin), OSENI (alogliptin/pioglitazone), JANUMET (sitagliptin/metformin), JANUMET XR (sitagliptin/metformin extended-release), JANUVIA (sitagliptin), JENTADUETO (linagliptin/metformin), JENTADUETO XR (linagliptin/metformin extended-release), KAZANO (alogliptin/metformin), KOMBIGLYZE XR (saxagliptin/metformin extended-release), NESINA (alogliptin), ONGLYZA (saxagliptin), OSENI (alogliptin/pioglitazone) TRADJENTA (linagliptin), JANUVIA (sitagliptin) DRUG CLASS: Dipeptidyl Peptidase-4 Inhibitor-(Biguanide Combinations), DPP-4 Inhibitor- Thiazolidinedione Combinations ROUTE OF ADMINISTRATION: Oral PLACE OF SERVICE: Retail Pharmacy AVAILABLE DOSAGE FORMS: Alogliptin Benzoate TABS 12.5MG,Alogliptin Benzoate TABS 25MG, Alogliptin Benzoate TABS 6.25MG, Alogliptin-Metformin HCl TABS 12.5-1000MG Alogliptin-Metformin HCl TABS 12.5-500MG, Janumet TABS 50-1000MG, Janumet TABS 50- 500MG, Janumet XR TB24 100-1000MG, Janumet XR TB24 50-1000MG, Janumet XR TB24 50- 500MG, Januvia TABS 100MG, Januvia TABS 25MG, Januvia TABS 50MG, Jentadueto TABS 2.5- 1000MG, Jentadueto TABS 2.5-500MG, Jentadueto TABS 2.5-500MG, Jentadueto TABS 2.5- 850MG, Jentadueto -

Polyhexamethylene Biguanide Hydrochloride) As Used in Cosmetics
Safety Assessment of Polyaminopropyl Biguanide (polyhexamethylene biguanide hydrochloride) as Used in Cosmetics Status: Tentative Report for Public Comment Release Date: September 26, 2017 Panel Date: December 4-5, 2017 All interested persons are provided 60 days from the above date to comment on this safety assessment and to identify additional published data that should be included or provide unpublished data which can be made public and included. Information may be submitted without identifying the source or the trade name of the cosmetic product containing the ingredient. All unpublished data submitted to CIR will be discussed in open meetings, will be available at the CIR office for review by any interested party and may be cited in a peer-reviewed scientific journal. Please submit data, comments, or requests to the CIR Executive Director, Dr. Bart Heldreth. The 2017 Cosmetic Ingredient Review Expert Panel members are: Chair, Wilma F. Bergfeld, M.D., F.A.C.P.; Donald V. Belsito, M.D.; Ronald A. Hill, Ph.D.; Curtis D. Klaassen, Ph.D.; Daniel C. Liebler, Ph.D.; James G. Marks, Jr., M.D.; Ronald C. Shank, Ph.D.; Thomas J. Slaga, Ph.D.; and Paul W. Snyder, D.V.M., Ph.D. The CIR Executive Director is Bart Heldreth, Ph.D. This report was prepared by Wilbur Johnson, Jr., M.S., Senior Scientific Analyst and Ivan Boyer, Ph.D., former Senior Toxicologist. © Cosmetic Ingredient Review 1620 L STREET, NW, SUITE 1200 ◊ WASHINGTON, DC 20036-4702 ◊ PH 202.331.0651 ◊ FAX 202.331.0088 ◊ [email protected] ABSTRACT: The Cosmetic Ingredient Review (CIR) Expert Panel (Panel) reviewed the safety of Polyaminopropyl Biguanide (polyhexamethylene biguanide hydrochloride), which functions as a preservative in cosmetic products. -

Effect of Exogenous Glucocorticoid on Osmotically Stimulated Antidiuretic
European Journal of Endocrinology (2006) 155 845–848 ISSN 0804-4643 CLINICAL STUDY Effect of exogenous glucocorticoid on osmotically stimulated antidiuretic hormone secretion and on water reabsorption in man Volker Ba¨hr1, Norma Franzen1, Wolfgang Oelkers2, Andreas F H Pfeiffer1 and Sven Diederich1,2 1Department of Endocrinology, Diabetes and Nutrition, Charite-Universitatsmedizin Berlin, Campus Benjamin Franklin, Hindenburgdamm 30, 12200 Berlin, Germany and 2Endokrinologikum Berlin, Centre for Endocrine and Metabolic Diseases, Berlin, Germany (Correspondence should be addressed to V Ba¨hr; Email: [email protected]) Abstract Objective: Glucocorticoids exert tonic suppression of antidiuretic hormone (ADH) secretion. Hypocortisolism in secondary adrenocortical insufficiency can result in a clinical picture similar to the syndrome of inappropriate ADH secretion. On the other hand, in vitro and in vivo results provide evidence for ADH suppression in states of hypercortisolism. To test the hypothesis that ADH suppression is of relevance during glucocorticoid therapy, we investigated the influence of prednisolone on the osmotic stimulation of ADH. Design and methods: Seven healthy men were subjected to water deprivation tests with the measurement of plasma ADH (pADH) and osmolality (posmol) before and after glucocorticoid treatment (5 days 30 mg prednisolone per day). Results: Before glucocorticoid treatment, the volunteers showed a normal test with an adequate increase of pADH (basal 0.54G0.2 to 1.9G0.72 pg/ml (meanGS.D.)) in relation to posmol(basal 283.3G8.5 to 293.7G6 mosmol/kg). After prednisolone intake, pADH was attenuated (!0.4 pg/ml) in spite of an increase of posmol from 289.3G3.6 to 297.0G5.5 mosmol/kg. -

A Review on Evolution in Triglyceride Determination
Available online at www.derpharmachemica.com ISSN 0975-413X Der Pharma Chemica, 2018, 10(5): 84-88 CODEN (USA): PCHHAX (http://www.derpharmachemica.com/archive.html) Sodium-glucose co-transporter 2 (SGLT2) Inhibitors: New Target for Type 2 Diabetes Mellitus (T2DM) Review Swapna Vadlamani* Asst. proffesor, NIPER, Hyderabad, Andhra Pradesh, India ABSTRACT Introduction: Knocking out type2 diabetes by new insulin independent renal glucose transporters as targets, reducing the side effects related to high rise in glucose levels is a more efficient way to manage diabetes. Sodium-glucose co-transporter 2 (SGLT2) inhibitors block reabsorption of glucose back into the blood and stimulate secretion in urine in a way controlling blood glucose levels. Areas discussed: We emphasize in this review an overview of type 2 diabetes. New insulin independent targets, SGLT family inhibitors and their mechanism of action are briefly discussed. Molecular modeling studies carried out for new analogues of SGLT2 were indicated and also about current marketed SGLT drugs their safety issues are briefly outlined. Conclusion: SGLT2 inhibitors are very promising drugs for near future, where insulin sensitization is a problem. A combination of drugs related to insulin dependent pathway and also independent pathway like SGLT2/SGLT1 drugs will be more effective in glycemic control with lesser side effects. Keywords: Type II Diabetes, SGLT2 inhibitors INTRODUCTION Present scenario of food habits and absolutely very less physical activity is becoming the major cause for obesity finally leading to diabetes. Diabetes is said to be a rich man disease and mostly occurs at the later age of 40, but now one in every 5 persons at early age diagnosed turned to be diabetic mainly because of lifestyle changes. -

Type 2 Diabetes Treatment Recommendations Update
abetes & Di M f e o t a l b a o Cornell, J Diabetes Metab 2014, 5:8 n l r i s u m o DOI: 10.4172/2155-6156.1000414 J Journal of Diabetes and Metabolism ISSN: 2155-6156 Review Article Open Access Type 2 Diabetes Treatment Recommendations Update: Appropriate Use of Dipeptidyl Peptidase-4 Inhibitors Susan Cornell* Midwestern University, Chicago College of Pharmacy, Downers Grove, IL, USA Abstract In this article, recommendations from the 2012 American Diabetes Association (ADA)/European Association for the Study of Diabetes (EASD) position statement are discussed with an emphasis on the appropriate use of Dipeptidyl Peptidase-4 (DPP-4) inhibitors in individuals with Type 2 Diabetes Mellitus (T2DM). The 2012 ADA/EASD position statement emphasizes individualization of treatment, with glycated hemoglobin (A1C) targets being determined for each patient based on life expectancy, complications, disease duration, comorbidities, such as cardiovascular disease or cognitive impairment, and the risk of hypoglycemia and other adverse events. Patients’ attitudes and support systems should also be considered. Recommendations for pharmacotherapy are less prescriptive and should be based on a patient’s needs, preferences, and tolerances. In general, metformin is recommended as first- line therapy for most patients, although combination of 2 noninsulin agents or insulin alone should be considered in patients with baseline A1C ≥ 9.0%. Add-on therapy to metformin will likely be needed to achieve and maintain glycemic control as the disease progresses. It is important to avoid therapies that increase the risk of weight gain or and, especially in older patients, hypoglycemia. As discussed in this review, DPP-4 inhibitors are well tolerated and effectively lower A1C and improve β-cell function without increasing the risk of hypoglycemia and weight gain. -

Beta-Blockers for Hypertension: Time to Call a Halt
Journal of Human Hypertension (1998) 12, 807–810 1998 Stockton Press. All rights reserved 0950-9240/98 $12.00 http://www.stockton-press.co.uk/jhh FOR DEBATE Beta-blockers for hypertension: time to call a halt DG Beevers University Department of Medicine, City Hospital, Birmingham B18 7QH, UK Beta-blockers are not very effective at lowering blood the endorsement of beta-blockers by the British Hyper- pressure in elderly hypertensive patients or in Afro- tension Society and other guidelines committees, Caribbeans and these two groups represent a large pro- except possibly for severe resistant hypertension, high portion of people with raised blood pressure. Further- risk post-infarct patients and those with angina pectoris. more they do not prevent more heart attacks than the The time has come to move across to newer, safer, more thiazide diuretics. Beta-blockers can also be dangerous tolerable and more effective antihypertensive agents in many hypertensive patients and even when these whilst continuing to use thiazide diuretics in low doses drugs are not contraindicated, they cause subtle and in the elderly as first choice, providing there are no depressing side effects which should preclude their contraindications. usefulness. The time has come therefore to reconsider Keywords: beta-blockers; hypertension Introduction Safety and tolerability Beta-adrenergic blockers were first introduced in the There is little doubt that the beta-blockers are the early 1960s for the treatment of angina pectoris. most unsafe of all antihypertensive drugs. They can Their antihypertensive properties were not fully precipitate or worsen heart failure in patients with recognised until the celebrated paper by Pritchard myocardial damage and they are contraindicated in and Gillam in 1964.1 They rapidly became popular patients with asthma. -

Glycemic Management of Type 2 Diabetes
Glycemic Management of Type 2 Diabetes Gail Nunlee-Bland, M.D. Professor Medicine & Pediatrics Director, Diabetes Treatment Center Howard University 1 Disclosures • None Learning Objectives • Understand the importance of lifestyle therapy in diabetes management • Know the classes of antihyperglycemic agents, mechanism of action, benefits and side effects of these agents • Recognize the importance of individualized treatment goals for diabetic patients AACE Comprehensive Care Plan Disease management from Antihyperglycemic a multidisciplinary team pharmacotherapy Comprehensive Care Plan Comprehensive diabetes Therapeutic lifestyle self-education for the change patient 4 Handelsman YH, et al. Endocr Pract. 2015;21(suppl 1):1-87. Glycemic Management of Type 2 Diabetes THERAPEUTIC LIFESTYLE CHANGE 5 Components of Therapeutic Lifestyle Change • Healthful eating • Sufficient physical activity • Sufficient sleep • Avoidance of tobacco products • Limited alcohol consumption • Stress reduction 6 Handelsman YH, et al. Endocr Pract. 2015;21(suppl 1):1-87. Glycemic Management of Type 2 Diabetes ANTIHYPERGLYCEMIC THERAPY 7 Cardiovascular Outcomes Trials: A Brief History • 2008 FDA guidance mandating assessment of CV safety of all antihyperglycemic agents in RCTs – Designed as noninferiority studies to demonstrate study drug was not associated with more MACE than placebo • Some study designs tested for superiority if noninferiority criteria were met – Primary endpoint: composite of cardiovascular death, nonfatal MI, and nonfatal stroke • Some primary -
Initiation Titration Assess Monitoring **
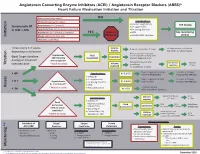
Angiotensin Converting Enzyme Inhibitors (ACEI) / Angiotensin Receptor Blockers (ARBS)* Heart Failure Medication Initiation and Titration Bilateral renal artery stenosis NO Moderate/Severe aortic stenosis Considerations: • Baseline cough (ACEi) Hyperkalemia: K+ > 5.2 mmol/L See dosing Symptomatic HF • K+ supplements or LVEF < 40% Renal Dysfunction:Serum creatinine >220 µmol/L • K+ sparing diuretics Hypotension: SBP < 90 mmHg or symptoms YES Refer to • MRA See monitoring Initiation Allergy: angioedema, hives, rash Physician • NSAIDS/COX2 inhibitors section Intolerance: cough (ACEi) Titrate every 1-3 weeks, Volume Reduce/hold diuretic x 2-3 days No improvement, hold/reduce depending on tolerance deplete ACEI/ARB x 1-2 wks & reassess Reassess diuretic dose/other Hypotension Fluid non-essential BP lowering meds Goal: Target dose(see Euvolemic SBP<90mmHg Assessment Consider staggering doses dosing) or maximum with symptoms* Reduce/hold dose of other See Diuretic Reassess Titration * Watch for trends Volume tolerated dose vasodilators algorithm 1-2 wks overload +/- ACEI/ARB x 1-2 weeks Stop K+ supplements, reduce/ Serum K+ in 3-5 days, Considerations: • BP K+ 5.2-5.5 hold MRA (if applicable) Reassess ACEI/ARB dose • Dietary K+ • K+ supplements Stop K+ supplements, MRA. Serum K+ in 2-3 days, • K + Hyperkalemia • K+ sparing diuretics K+ 5.6-6.0 hold ACEI/ARB Reassess ACEI/ARB dose K+ > 5.2 mmol/L* • MRA Assess * Watch for trends • Renal dysfunction Treat hyperkalemia • Scr K+ > 6.0 Refer to MD/NP +/- send to ED Reduce/hold diuretic Scr in Considerations: -

Energy Drinks 800.232.4424 (Voice/TTY) 860.793.9813 (Fax)
Caffeine and Energy Boosting Drugs: Energy Drinks 800.232.4424 (Voice/TTY) 860.793.9813 (Fax) www.ctclearinghouse.org A Library and Resource Center on Alcohol, Tobacco, Other Drugs, Mental Health and Wellness What are energy drinks? you. You wouldn't use Mountain Dew as a sports Energy drinks are beverages like drink. And a drink like Red Bull and vodka is Red Bull, Venom, Adrenaline Rush, more like strong coffee and whisky than 180, ISO Sprint, and Whoopass, anything else. which contain large doses of caffeine and other legal stimulants What happens when energy drinks are like ephedrine, guarana, and ginseng. combined with alcohol? Energy drinks may contain as much as 80 mg. Energy drinks are also used as mixers with of caffeine, the equivalent of a cup of coffee. alcohol. This combination carries a number of Compared to the 37 mg. of caffeine in a dangers: Mountain Dew, or the 23 mg. in a Coca-Cola Classic, that's a big punch. These drinks are • Since energy drinks are stimulants and marketed to people under 30, especially to alcohol is a depressant, the combination of college students, and are widely available both effects may be dangerous. The stimulant on and off campus. effects can mask how intoxicated you are and prevent you from realizing how much Are there short-term dangers to drinking alcohol you have consumed. Fatigue is one energy drinks? of the ways the body normally tells Individual responses to caffeine vary, and someone that they've had enough to drink. these drinks should be treated carefully • The stimulant effect can give the person the because of how powerful they are. -

Dipeptidyl Peptidase-IV Inhibitors: Pharmacological Profile and Clinical Use
F E A T U R E A R TICLE Dipeptidyl Peptidase-IV Inhibitors: Pharmacological Profile and Clinical Use John R. White, Jr., PA, PharmD decade and a half ago, the on insulin biosynthesis and its inhibition creation of molecules that circumvent or choice of an oral antihypergly- of glucagon release.2 reduce the rate degradation by DPP-IV Acemic agent for any particu- Because GLP-1 stimulates insulin while maintaining the agonist effects of lar patient was in some ways a debate secretion only under hyperglycemic GLP-1 has been pursued aggressively. of nuance. Sulfonylureas (SUs) were conditions, there is minimal risk of One GLP-1 analog (exenatide) is avail- the only class available, and provid- hypoglycemia, making this molecule and able in the United States, and another ers were left to sift though pharmacoki- its congeners likely candidates for use as (liraglutide) is in phase III trials. netic, small efficacy, and sometimes sig- antihyperglycemic agents. GLP-1 is also In addition to GLP-1 analogs, mol- nificant side effect differences among associated with increased satiety, possi- ecules that inhibit the activity of DPP-IV the various choices within this class. The bly because it reduces the rate of gastric and thereby prolong the activity of situation today demands a more robust emptying. People with type 2 diabetes endogenous GLP-1 are of great interest. evaluation of multiple categories of med- have reduced circulating levels of GLP-1 Because GLP-1 analogs are proteina- ications with different mechanisms of but retain their ability to respond to this cious in structure, they will most likely action.