Comparison of Effects Between Calcium Channel Blocker and Diuretics in Combination with Angiotensin II Receptor Blocker on 24-H
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Still an Important Cause of Heart Failure?
Journal of Human Hypertension (2005) 19, 267–275 & 2005 Nature Publishing Group All rights reserved 0950-9240/05 $30.00 www.nature.com/jhh REVIEW ARTICLE Hypertension — still an important cause of heart failure? E Kazzam1, BA Ghurbana2, EN Obineche1 and MG Nicholls1 1Department of Internal Medicine, Faculty of Medicine and Health Sciences, UAE University, Al Ain, United Arab Emirates; 2Department of Medicine, Al Ain Hospital, Al Ain, United Arab Emirates Hypertension has been the single most important risk from highly selected study groups in tertiary referral factor for heart failure until the last few decades. Now, it centres to patients with heart failure in primary and is frequently claimed that atherosclerotic coronary secondary care, may not be justified. Finally, the artery disease dominates as the major underlying cause, situation of heart failure primarily due to impaired left and hypertension is of lesser importance. We here ventricular diastolic function, where hypertension is a review evidence regarding the contribution of hyperten- frequent precursor, is often ignored in discussions of sion to heart failure in the recent decades. It is not aetiology. Our view is that hypertension remains and possible, in our view, to be confident of the relative probably is the single most, important modifiable risk importance of hypertension and coronary artery disease factor for cardiac failure in some races and countries, since there are significant limitations in the available where the dominant cardiac abnormality is left ventri- data. The often-questionable diagnostic criteria used in cular diastolic dysfunction. The situation is less clear for defining heart failure is one such limitation. -

Association of Hypertensive Status and Its Drug Treatment with Lipid and Haemostatic Factors in Middle-Aged Men: the PRIME Study
Journal of Human Hypertension (2000) 14, 511–518 2000 Macmillan Publishers Ltd All rights reserved 0950-9240/00 $15.00 www.nature.com/jhh ORIGINAL ARTICLE Association of hypertensive status and its drug treatment with lipid and haemostatic factors in middle-aged men: the PRIME Study P Marques-Vidal1, M Montaye2, B Haas3, A Bingham4, A Evans5, I Juhan-Vague6, J Ferrie`res1, G Luc2, P Amouyel2, D Arveiler3, D McMaster5, JB Ruidavets1, J-M Bard2, PY Scarabin4 and P Ducimetie`re4 1INSERM U518, Faculte´ de Me´decine Purpan, Toulouse, France; 2MONICA-Lille, Institut Pasteur de Lille, Lille, France; 3MONICA-Strasbourg, Laboratoire d’Epide´miologie et de Sante´ Publique, Strasbourg, France; 4INSERM U258, Hoˆ pital Broussais, Paris, France; 5Belfast-MONICA, Department of Epidemiology, The Queen’s University of Belfast, UK; 6Laboratory of Haematology, La Timone Hospital, Marseille, France Aims: To assess the association of hypertensive status this effect remained after multivariate adjustment. Cal- and antihypertensive drug treatment with lipid and hae- cium channel blockers decreased total cholesterol and mostatic levels in middle-aged men. apoproteins A-I and B; those differences remained sig- Methods and results: Hypertensive status, antihyperten- nificant after multivariate adjustment. ACE inhibitors sive drug treatment, total and high-density lipoprotein decreased total cholesterol, triglycerides, apoprotein B (HDL) cholesterol, triglyceride, apoproteins A-I and B, and LpE:B; and this effect remained after multivariate lipoparticles LpA-I, -

Effect of Exogenous Glucocorticoid on Osmotically Stimulated Antidiuretic
European Journal of Endocrinology (2006) 155 845–848 ISSN 0804-4643 CLINICAL STUDY Effect of exogenous glucocorticoid on osmotically stimulated antidiuretic hormone secretion and on water reabsorption in man Volker Ba¨hr1, Norma Franzen1, Wolfgang Oelkers2, Andreas F H Pfeiffer1 and Sven Diederich1,2 1Department of Endocrinology, Diabetes and Nutrition, Charite-Universitatsmedizin Berlin, Campus Benjamin Franklin, Hindenburgdamm 30, 12200 Berlin, Germany and 2Endokrinologikum Berlin, Centre for Endocrine and Metabolic Diseases, Berlin, Germany (Correspondence should be addressed to V Ba¨hr; Email: [email protected]) Abstract Objective: Glucocorticoids exert tonic suppression of antidiuretic hormone (ADH) secretion. Hypocortisolism in secondary adrenocortical insufficiency can result in a clinical picture similar to the syndrome of inappropriate ADH secretion. On the other hand, in vitro and in vivo results provide evidence for ADH suppression in states of hypercortisolism. To test the hypothesis that ADH suppression is of relevance during glucocorticoid therapy, we investigated the influence of prednisolone on the osmotic stimulation of ADH. Design and methods: Seven healthy men were subjected to water deprivation tests with the measurement of plasma ADH (pADH) and osmolality (posmol) before and after glucocorticoid treatment (5 days 30 mg prednisolone per day). Results: Before glucocorticoid treatment, the volunteers showed a normal test with an adequate increase of pADH (basal 0.54G0.2 to 1.9G0.72 pg/ml (meanGS.D.)) in relation to posmol(basal 283.3G8.5 to 293.7G6 mosmol/kg). After prednisolone intake, pADH was attenuated (!0.4 pg/ml) in spite of an increase of posmol from 289.3G3.6 to 297.0G5.5 mosmol/kg. -

Rational Use of Beta Blockers in Management of Hypertension Khawaja Tahir Mehmood, Fatima Amin, Hafiza Kiran Ismail, Ayesha Irfan
Khawaja Tahir Mahmood et al /J. Pharm. Sci. & Res. Vol.3(1), 2011,1029-1034 Rational Use of Beta Blockers in Management of Hypertension Khawaja Tahir Mehmood, Fatima Amin, Hafiza Kiran Ismail, Ayesha Irfan. Lahore College for women university Lahore, Pakistan Abstract: Hypertension is an extremely common disorder. It is major risk factor for cardiovascular morbidity and mortality through its effects on target organs like brain heart kidney eyes. In Pakistan magnitude of problem of uncontrolled hypertension is greater. .Treatment of hypertension decreases morbidity and increases life expectancy. Beta blockers are widely used in the treatment of hypertension. Beta blockers are effective only when they are rationally prescribed and used. It is retrospective type of study during which data of 50 hypertensive patients with primary hypertension using beta blockers were collected to analyze whether beta blockers are rationally prescribed and used. After therapy with beta blockers Blood pressure is effectively controlled. After the therapy 20 % patient have B.P in range of 110-130, 56% patients have B.P in range of 120- 130 and 24% have B.P in range of 130-140.It is concluded that blood pressure is effectively controlled when beta blockers rationally used. It should be given with caution in older and diabetic patients because it increases the risk of stroke in older patients. It is most effective when given in combination. Key words; Beta blockers, Hypertension, rational use, INTRODUCTION: systolic hypertension, white coat hypertension Hypertension is defined as either a sustained and resistant hypertension. (5, 6) systolic blood pressure of greater than 140 Although exact causes of primary hypertension mmHg or sustained diastolic blood pressure of is not fully understood it is known that some greater than 90mmHg. -

Beta-Blockers for Hypertension: Time to Call a Halt
Journal of Human Hypertension (1998) 12, 807–810 1998 Stockton Press. All rights reserved 0950-9240/98 $12.00 http://www.stockton-press.co.uk/jhh FOR DEBATE Beta-blockers for hypertension: time to call a halt DG Beevers University Department of Medicine, City Hospital, Birmingham B18 7QH, UK Beta-blockers are not very effective at lowering blood the endorsement of beta-blockers by the British Hyper- pressure in elderly hypertensive patients or in Afro- tension Society and other guidelines committees, Caribbeans and these two groups represent a large pro- except possibly for severe resistant hypertension, high portion of people with raised blood pressure. Further- risk post-infarct patients and those with angina pectoris. more they do not prevent more heart attacks than the The time has come to move across to newer, safer, more thiazide diuretics. Beta-blockers can also be dangerous tolerable and more effective antihypertensive agents in many hypertensive patients and even when these whilst continuing to use thiazide diuretics in low doses drugs are not contraindicated, they cause subtle and in the elderly as first choice, providing there are no depressing side effects which should preclude their contraindications. usefulness. The time has come therefore to reconsider Keywords: beta-blockers; hypertension Introduction Safety and tolerability Beta-adrenergic blockers were first introduced in the There is little doubt that the beta-blockers are the early 1960s for the treatment of angina pectoris. most unsafe of all antihypertensive drugs. They can Their antihypertensive properties were not fully precipitate or worsen heart failure in patients with recognised until the celebrated paper by Pritchard myocardial damage and they are contraindicated in and Gillam in 1964.1 They rapidly became popular patients with asthma. -
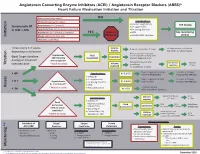
Initiation Titration Assess Monitoring **
Angiotensin Converting Enzyme Inhibitors (ACEI) / Angiotensin Receptor Blockers (ARBS)* Heart Failure Medication Initiation and Titration Bilateral renal artery stenosis NO Moderate/Severe aortic stenosis Considerations: • Baseline cough (ACEi) Hyperkalemia: K+ > 5.2 mmol/L See dosing Symptomatic HF • K+ supplements or LVEF < 40% Renal Dysfunction:Serum creatinine >220 µmol/L • K+ sparing diuretics Hypotension: SBP < 90 mmHg or symptoms YES Refer to • MRA See monitoring Initiation Allergy: angioedema, hives, rash Physician • NSAIDS/COX2 inhibitors section Intolerance: cough (ACEi) Titrate every 1-3 weeks, Volume Reduce/hold diuretic x 2-3 days No improvement, hold/reduce depending on tolerance deplete ACEI/ARB x 1-2 wks & reassess Reassess diuretic dose/other Hypotension Fluid non-essential BP lowering meds Goal: Target dose(see Euvolemic SBP<90mmHg Assessment Consider staggering doses dosing) or maximum with symptoms* Reduce/hold dose of other See Diuretic Reassess Titration * Watch for trends Volume tolerated dose vasodilators algorithm 1-2 wks overload +/- ACEI/ARB x 1-2 weeks Stop K+ supplements, reduce/ Serum K+ in 3-5 days, Considerations: • BP K+ 5.2-5.5 hold MRA (if applicable) Reassess ACEI/ARB dose • Dietary K+ • K+ supplements Stop K+ supplements, MRA. Serum K+ in 2-3 days, • K + Hyperkalemia • K+ sparing diuretics K+ 5.6-6.0 hold ACEI/ARB Reassess ACEI/ARB dose K+ > 5.2 mmol/L* • MRA Assess * Watch for trends • Renal dysfunction Treat hyperkalemia • Scr K+ > 6.0 Refer to MD/NP +/- send to ED Reduce/hold diuretic Scr in Considerations: -

Energy Drinks 800.232.4424 (Voice/TTY) 860.793.9813 (Fax)
Caffeine and Energy Boosting Drugs: Energy Drinks 800.232.4424 (Voice/TTY) 860.793.9813 (Fax) www.ctclearinghouse.org A Library and Resource Center on Alcohol, Tobacco, Other Drugs, Mental Health and Wellness What are energy drinks? you. You wouldn't use Mountain Dew as a sports Energy drinks are beverages like drink. And a drink like Red Bull and vodka is Red Bull, Venom, Adrenaline Rush, more like strong coffee and whisky than 180, ISO Sprint, and Whoopass, anything else. which contain large doses of caffeine and other legal stimulants What happens when energy drinks are like ephedrine, guarana, and ginseng. combined with alcohol? Energy drinks may contain as much as 80 mg. Energy drinks are also used as mixers with of caffeine, the equivalent of a cup of coffee. alcohol. This combination carries a number of Compared to the 37 mg. of caffeine in a dangers: Mountain Dew, or the 23 mg. in a Coca-Cola Classic, that's a big punch. These drinks are • Since energy drinks are stimulants and marketed to people under 30, especially to alcohol is a depressant, the combination of college students, and are widely available both effects may be dangerous. The stimulant on and off campus. effects can mask how intoxicated you are and prevent you from realizing how much Are there short-term dangers to drinking alcohol you have consumed. Fatigue is one energy drinks? of the ways the body normally tells Individual responses to caffeine vary, and someone that they've had enough to drink. these drinks should be treated carefully • The stimulant effect can give the person the because of how powerful they are. -

Comparison of Effectiveness and Safety of Labetalol and Hydralazine on Blood Pressure During Cataract Surgery in Patients with Hypertensio
Revista Latinoamericana de Hipertensión ISSN: 1856-4550 [email protected] Sociedad Latinoamericana de Hipertensión Venezuela Comparison of effectiveness and safety of labetalol and hydralazine on blood pressure during cataract surgery in patients with hypertensio Espahbodi, Ebrahim; Takzare, Alireza; Sanatkar, Mehdi; Goudarzi, Mehrdad Comparison of effectiveness and safety of labetalol and hydralazine on blood pressure during cataract surgery in patients with hypertensio Revista Latinoamericana de Hipertensión, vol. 14, no. 4, 2019 Sociedad Latinoamericana de Hipertensión, Venezuela Available in: https://www.redalyc.org/articulo.oa?id=170263002018 Derechos reservados. Queda prohibida la reproducción total o parcial de todo el material contenido en la revista sin el consentimiento por escrito del editor en jefe. Copias de los artículos: Todo pedido de separatas deberá ser gestionado directamente con el editor en jefe, quien gestionará dicha solicitud ante la editorial encargada de la publicación. This work is licensed under Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International. PDF generated from XML JATS4R by Redalyc Project academic non-profit, developed under the open access initiative Revista Latinoamericana de Hipertensión, 2019, vol. 14, no. 4, ISSN: 1856-4550 Artículos Comparison of effectiveness and safety of labetalol and hydralazine on blood pressure during cataract surgery in patients with hypertensio Comparación de la efectividad y la seguridad de labetalol e hidralazina en la presión arterial -

The Necessity and Effectiveness of Mineralocorticoid Receptor Antagonist in the Treatment of Diabetic Nephropathy
Hypertension Research (2015) 38, 367–374 & 2015 The Japanese Society of Hypertension All rights reserved 0916-9636/15 www.nature.com/hr REVIEW The necessity and effectiveness of mineralocorticoid receptor antagonist in the treatment of diabetic nephropathy Atsuhisa Sato Diabetes mellitus is a major cause of chronic kidney disease (CKD), and diabetic nephropathy is the most common primary disease necessitating dialysis treatment in the world including Japan. Major guidelines for treatment of hypertension in Japan, the United States and Europe recommend the use of angiotensin-converting enzyme inhibitors and angiotensin-receptor blockers, which suppress the renin-angiotensin system (RAS), as the antihypertensive drugs of first choice in patients with coexisting diabetes. However, even with the administration of RAS inhibitors, failure to achieve adequate anti-albuminuric, renoprotective effects and a reduction in cardiovascular events has also been reported. Inadequate blockade of aldosterone may be one of the reasons why long-term administration of RAS inhibitors may not be sufficiently effective in patients with diabetic nephropathy. This review focuses on treatment in diabetic nephropathy and discusses the significance of aldosterone blockade. In pre-nephropathy without overt nephropathy, a mineralocorticoid receptor antagonist can be used to enhance the blood pressure-lowering effects of RAS inhibitors, improve insulin resistance and prevent clinical progression of nephropathy. In CKD categories A2 and A3, the addition of a mineralocorticoid receptor antagonist to an RAS inhibitor can help to maintain ‘long-term’ antiproteinuric and anti-albuminuric effects. However, in category G3a and higher, sufficient attention must be paid to hyperkalemia. Mineralocorticoid receptor antagonists are not currently recommended as standard treatment in diabetic nephropathy. -

Optimal Diuretic Strategies in Heart Failure
517 Review Article on Heart Failure Update and Advances in 2021 Page 1 of 8 Optimal diuretic strategies in heart failure Sarabjeet S. Suri, Salpy V. Pamboukian Division of Cardiovascular Diseases, University of Alabama at Birmingham, Birmingham, AL, USA Contributions: (I) Conception and design: SS Suri; (II) Administrative support: SV Pamboukian; (III) Provision of study materials or patients: Both authors; (IV) Collection and assembly of data: Both authors; (V) Data analysis and interpretation: Both authors; (VI) Manuscript writing: Both authors; (VII) Final approval of manuscript: Both authors. Correspondence to: Dr. Sarabjeet S. Suri, MD. University of Alabama at Birmingham, 1900 University Blvd, THT 321, Birmingham, AL 35223, USA. Email: [email protected]. Abstract: Heart failure (HF) is one of the major causes of morbidity and mortality in the world. According to a 2019 American Heart Association report, about 6.2 million American adults had HF between 2013 and 2016, being responsible for almost 1 million admissions. As the population ages, the prevalence of HF is anticipated to increase, with 8 million Americans projected to have HF by 2030, posing a significant public health and financial burden. Acute decompensated HF (ADHF) is a syndrome characterized by volume overload and inadequate cardiac output associated with symptoms including some combination of exertional shortness of breath, orthopnea, paroxysmal nocturnal dyspnea (PND), fatigue, tissue congestion (e.g., peripheral edema) and decreased mentation. The pathology is characterized by hemodynamic abnormalities that result in autonomic imbalance with an increase in sympathetic activity, withdrawal of vagal activity and neurohormonal activation (NA) resulting in increased plasma volume in the setting of decreased sodium excretion, increased water retention and in turn an elevation of filling pressures. -

Timeline of History of Hypertension Treatment
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Frontiers - Publisher Connector REVIEW published: 23 February 2016 doi: 10.3389/fcvm.2016.00003 Timeline of History of Hypertension Treatment Mohammad G. Saklayen1* and Neeraj V. Deshpande2 1V.A. Medical Center, Wright State University Boonshoft School of Medicine, Dayton, OH, USA, 2Ohio State University, Columbus, OH, USA It is surprising that only about 50 years ago hypertension was considered an essential malady and not a treatable condition. Introduction of thiazide diuretics in late 50s made some headway in successful treatment of hypertension and ambitious multicenter VA co-operative study (phase 1 and 2) started in 1964 for diastolic hypertension ranging between 90 and 129 mmHg and completed by 1971 established for the first time that treating diastolic hypertension reduced CV events such as stroke and heart failure and improved mortality. In the following decade, these results were confirmed for the wider US and non-US population, including women and goal-oriented BP treatment to diastolic 90 became the standard therapy recommendation. But isolated systolic hypertension (accounting for two-thirds of the 70 million hypertensive population in USA alone) was not considered treatable until 1991 when SHEP study (systolic hypertension in elderly program) was completed and showed tremendous benefits of treating systolic BP over 160 mmHg using only a simple regimen using small dose chlorthalidone with Edited by: addition of atenolol if needed. In the next two decades, ALLHAT and other studies Claudio Letizia, examined the comparability of outcomes with use of different classes and combinations Sapienza University of Rome, Italy of antihypertensive drugs. -

Historical Perspective
Journal of Integrated Community Health Volume 9, Issue 1 - 2020, Pg. No. 31-34 Peer Reviewed & Open Access Journal Review Article Milestones in Understanding and Management of Hypertension through Ages: Historical Perspective Mohd Javaid1, SM Safdar Ashraf2, Abdul Aziz Khan3 1PG Scholar, 2Professor, 3Assistant Professor, Department of Tahaffuzi wa Samaji Tib, Ajmal Khan Tibbiya College, Faculty of Unani Medicine, Aligarh Muslim University, Aligarh, India. INFO ABSTRACT Corresponding Author: The history of hypertension goes back a long way. Ancient Chinese and Mohd Javaid, Department of Tahaffuzi wa Samaji Indian Physicians felt the quality of an individual’s pulse by palpation Tib, Ajmal Khan Tibbiya College, Faculty of Unani and considered it as an indicator for the conditions of the cardiovascular Medicine, Aligarh Muslim University, Aligarh, system. What was called “hard pulse” possibly would qualify for the India. modern term of hypertension. E-mail Id: Frederick Akbar Mahomed, an Irish-Indian physician, first described [email protected] condition that known as “essential hypertension” separating it from Orcid Id: vascular changes that founds in chronic glomerulonephritis such as https://orcid.org/0000-0002-6786-1337 Bright’s disease, in nineteenth century. He also described that high How to cite this article: Blood pressure may be found in apparently healthy persons, that high Javaid M, Ashraf SMS, Khan AA. Milestones in blood pressure was more exist in old persons and that kidney; heart Understanding and Management of Hypertension and brain may be affected by high arterial tension.21,22 through Ages: Historical Perspective. J Integ Comm Health 2020; 9(1): 31-34. In the early twentieth century, mercury sphygmomanometer was invented and systolic and diastolic blood pressure were defined by Date of Submission: 2020-03-05 appearance/ disappearance of Korotkoff sounds as heard via the Date of Acceptance: 2020-05-06 stethoscope, and then present quantitative idea of hypertension means systolic and diastolic categories existed.