Effects of Glucose and Glucoregulatory Hormones
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Supporting Information
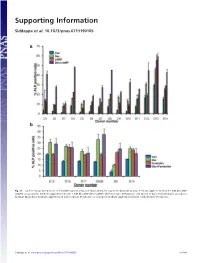
Supporting Information Siddappa et al. 10.1073/pnas.0711190105 Fig. S1. (a) Percentage ALP-positive cells in hMSCs grown in basic medium (Con), osteogenic medium (Dex), basic medium supplemented with 1 mM db-cAMP (cAMP), or osteogenic medium supplemented with 1 mM db-cAMP (DexϩcAMP). (b) Percentage ALP-positive cells grown in basic medium (Con), osteogenic medium (Dex), basic medium supplemented with forskolin (Forskolin), or osteogenic medium supplemented with forskolin (DexϩForskolin). Siddappa et al. www.pnas.org/cgi/content/short/0711190105 1of10 Fig. S2. hMSCs were grown in basic medium, basic medium supplemented with 1 mM db-cAMP (cAMP), osteogenic medium (Dex), or osteogenic medium supplemented with 1 mM db-cAMP (DexϩcAMP). Expression was analyzed by qPCR and is expressed as fold induction compared with cells grown in basic medium. The data were analyzed by using two-way ANOVA, and statistical significance is indicated compared with cells grown in basic medium. *, P Ͻ 0.05. Siddappa et al. www.pnas.org/cgi/content/short/0711190105 2of10 Fig. S3. (a) Methylene blue staining of hMSC-seeded scaffolds grown in basic medium (Con) or basic medium supplemented with 1 mM db-cAMP (cAMP) for 4 days. Note the less intensely stained db-cAMP-treated construct, indicating reduced cell numbers. (b) Quantitative Alamar blue assay for cell number analysis. The data were analyzed by using one-way ANOVA followed by Dunnet’s multiple-comparison test. Statistical significance is indicated compared with cells grown in basic medium (Con). *, P Ͻ 0.05 Siddappa et al. www.pnas.org/cgi/content/short/0711190105 3of10 Fig. -

Feedback Regulation of Growth Hormone Synthesis and Secretion in Fish and the Emerging Concept of Intrapituitary Feedback Loop ☆ ⁎ Anderson O.L
http://www.paper.edu.cn Comparative Biochemistry and Physiology, Part A 144 (2006) 284–305 Review Feedback regulation of growth hormone synthesis and secretion in fish and the emerging concept of intrapituitary feedback loop ☆ ⁎ Anderson O.L. Wong , Hong Zhou, Yonghua Jiang, Wendy K.W. Ko Department of Zoology, University of Hong Kong, Pokfulam Road, Hong Kong, P.R. China Received 29 July 2005; received in revised form 21 November 2005; accepted 21 November 2005 Available online 9 January 2006 Abstract Growth hormone (GH) is known to play a key role in the regulation of body growth and metabolism. Similar to mammals, GH secretion in fish is under the control of hypothalamic factors. Besides, signals generated within the pituitary and/or from peripheral tissues/organs can also exert a feedback control on GH release by effects acting on both the hypothalamus and/or anterior pituitary. Among these feedback signals, the functional role of IGF is well conserved from fish to mammals. In contrast, the effects of steroids and thyroid hormones are more variable and appear to be species-specific. Recently, a novel intrapituitary feedback loop regulating GH release and GH gene expression has been identified in fish. This feedback loop has three functional components: (i) LH induction of GH release from somatotrophs, (ii) amplification of GH secretion by GH autoregulation in somatotrophs, and (iii) GH feedback inhibition of LH release from neighboring gonadotrophs. In this article, the mechanisms for feedback control of GH synthesis and secretion are reviewed and functional implications of this local feedback loop are discussed. This intrapituitary feedback loop may represent a new facet of pituitary research with potential applications in aquaculture and clinical studies. -

Table of Contents
Therapeutic systems for Insulin-like growth factor-I Dissertation zur Erlangung des naturwissenschaftlichen Doktorgrades der Julius-Maximilians-Universität Würzburg vorgelegt von Isabel Schultz aus Dahn Würzburg 2015 Eingereicht bei der Fakultät für Chemie und Pharmazie am Gutachter der schriftlichen Arbeit 1. Gutachter: 2. Gutachter: Prüfer des öffentlichen Promotionskolloquiums 1. Prüfer: 2. Prüfer: 3. Prüfer: Datum des öffentlichen Promotionskolloquiums Doktorurkunde ausgehändigt am TABLE OF CONTENTS TABLE OF CONTENTS SUMMARY ............................................................................... 1 ZUSAMMEMFASSUNG .......................................................... 5 CHAPTER I ............................................................................... 9 DRUG DELIVERY OF INSULIN-LIKE GROWTH FACTOR I CHAPTER II ............................................................................ 45 INSULIN-LIKE GROWTH FACTOR-I AEROSOL FORMULATIONS FOR PULMONARY DELIVERY CHAPTER III ........................................................................... 73 PULMONARY INSULIN-LIKE GROWTH FACTOR I DELIVERY FROM TREHALOSE AND SILK-FIBROIN MICROPARTICLES CHAPTER IV ......................................................................... 113 EXPRESSION OF IGF-I MUTANTS CONCLUSION AND OUTLOOK ......................................... 147 DOCUMENTATION OF AUTHORSHIP ............................. 159 CURRICULUM VITAE ......................................................... 163 ACKNOWLEDGMENTS ..................................................... -

Thymic Mesenchymal Cells Have a Distinct Transcriptomic Profile
Thymic Mesenchymal Cells Have a Distinct Transcriptomic Profile Julien Patenaude and Claude Perreault This information is current as J Immunol 2016; 196:4760-4770; Prepublished online 29 of October 1, 2021. April 2016; doi: 10.4049/jimmunol.1502499 http://www.jimmunol.org/content/196/11/4760 Downloaded from Supplementary http://www.jimmunol.org/content/suppl/2016/04/29/jimmunol.150249 Material 9.DCSupplemental References This article cites 65 articles, 18 of which you can access for free at: http://www.jimmunol.org/content/196/11/4760.full#ref-list-1 http://www.jimmunol.org/ Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication by guest on October 1, 2021 *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2016 by The American Association of Immunologists, Inc. All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. The Journal of Immunology Thymic Mesenchymal Cells Have a Distinct Transcriptomic Profile Julien Patenaude and Claude Perreault In order to understand the role of mesenchymal cells (MCs) in the adult thymus, we performed whole transcriptome analyses of primary thymic, bone, and skin MCs. -

Growth Hormone and Insulin-Like Growth Factors I and II Produce
Proc. Natl. Acad. Sci. USA Vol. 82, pp. 8724-8728, December 1985 Medical Sciences Growth hormone and insulin-like growth factors I and II produce distinct alterations in glucose metabolism in 3T3-F442A adipocytes (glucose oxidation/lipid accumulation/somatomedin C/multiplication-stimulating activity/recombinant DNA-derived insulin-like growth factor I) JESSICA SCHWARTZ, CAROL M. FOSTER, AND MARTA S. SATIN Department of Physiology, University of Michigan Medical School, Ann Arbor, MI 48109 Communicated by H. W. Davenport, August 20, 1985 ABSTRACT In 3T3-F442A adipocytes, human growth difficult (9, 10) because the onset of the suppressive effects hormone (hGH) stimulates glucose oxidation in 4 hr. A requires several days and in vitro preparations sensitive to maximal increase is evident at hGH concentrations of 50-100 GH (e.g., adipocytes) are not viable for extended periods. ng/ml and rarely exceeds 50% above control. The stimulation Recently, we reported that cultured 3T3-F442A adipocytes is transient; after 48 hr of incubation with GH, glucose could be used to study the metabolic effects of GH (11). The oxidation is significantly suppressed to 35% below control differentiation of 3T3-F442A fibroblasts to adipocytes has values. In view of the concept that insulin-like growth factors been shown to be GH-dependent (12), although GH is not (IGF) may mediate the effects of GH, we compared the effects required to maintain the adipocytes. We have found that, ofhGH (500 ng/ml) and several preparations ofIGF on glucose once converted, the 3T3 adipocytes are sensitive to both metabolism in 3T3 adipocytes. After 4 hr of incubation, IGF-I stimulatory and suppressive effects of human GH (hGH) on from human plasma stimulated glucose oxidation in a dose- glucose metabolism (11). -

Alternative Processing of Bovine Growth Hormone Mrna
Proc. Natl. Acad. Sci. USA Vol. 84, pp. 2673-2677, May 1987 Biochemistry Alternative processing of bovine growth hormone mRNA: Nonsplicing of the final intron predicts a high molecular weight variant of bovine growth hormone (intron D/alternative reading frame/growth hormone-related polypeptide) ROBERT K. HAMPSON AND FRITZ M. ROTTMAN Department of Molecular Biology and Microbiology, Case Western Reserve University School of Medicine, 2119 Abington Road, Cleveland, OH 44106 Communicated by Lester 0. Krampitz, January 2, 1987 (received for review September 10, 1986) ABSTRACT We have detected a variant species of bovine MATERIALS AND METHODS growth hormone mRNA in bovine pituitary tissue and in a stably transfected bovine growth hormone-producing cell line. CHO 14-10-4 Cell Line. The Chinese hamster ovary (CHO) Analysis of this variant mRNA indicated that the last inter- cell line, CHO 14-10-4, utilized in these studies was gener- vening sequence (intron D) had not been removed by splicing. ously provided by Leonard Post. These cells were derived Inspection of the sequence of intron D reveals an open reading frame through the entire intron, with a termination codon from the DBX-11 cell line of dihydrofolate reductase-nega- encountered 50 nucleotides into the fifth exon, which is shifted tive CHO cells (6) and have been stably transfected with an from the normal reading frame in this variant mRNA. If expression plasmid containing the bovine growth hormone translated, this variant mRNA would encode a growth hor- gene. This expression plasmid, pSV2Cdhfr (Fig. 1), contains mone-related polypeptide having 125 amino-terminal amino the BamHI/EcoRI fragment of the bovine growth hormone acids identical to wild-type growth hormone, followed by 108 genomic clone (2) in the plasmid pSV2dhfr (7) situated carboxyl-terminal amino acids encoded by the 274 bases of downstream from a 760-base-pair Sau3A fragment containing intron D along with the first 50 nucleotides of exon 5. -

Download?Doi=10.1.1.640.8406&Rep=Rep1&T Ype=Pdf
UC Riverside UC Riverside Electronic Theses and Dissertations Title The Effects of Temperature, Salinity, and Bifenthrin on the Behavior and Neuroendocrinology of Juvenile Salmon and Trout Permalink https://escholarship.org/uc/item/9jv04705 Author Giroux, Marissa Sarah Publication Date 2019 License https://creativecommons.org/licenses/by-nd/4.0/ 4.0 Peer reviewed|Thesis/dissertation eScholarship.org Powered by the California Digital Library University of California UNIVERSITY OF CALIFORNIA RIVERSIDE The Effects of Temperature, Salinity, and Bifenthrin on the Behavior and Neuroendocrinology of Juvenile Salmon and Trout A Dissertation submitted in partial satisfaction of the requirements for the degree of Doctor of Philosophy in Environmental Toxicology by Marissa S. Giroux June 2019 Dissertation Committee: Dr. Daniel Schlenk, Chairperson Dr. David Volz Dr. Andrew Gray Copyright by Marissa S. Giroux 2019 The Dissertation of Marissa S. Giroux is approved: Committee Chairperson University of California, Riverside ACKNOWLEDGEMENTS Without the support, guidance, training, and time of many people this dissertation would not be possible. First, I would like to thank my Advisor, Dr. Daniel Schlenk for his help in in applying for fellowships, guidance in experimental design, taking the time to help me network, and giving me the opportunity to teach and mentor in both the lab and classroom. I would also like to thank my committee members, Dr. David Volz and Dr. Andrew Gray, for their wonderful recommendations and advice throughout my years at UC-Riverside. I have immense gratitude for my lab family, both past and present, who trained me, supported me, and taught me all of the “other” things you learn in grad school. -

Growth Hormone Therapy in Children with Chronic Renal Failure
Eurasian J Med 2015; 47: 62-5 Review Growth Hormone Therapy in Children with Chronic Renal Failure Kronik Böbrek Yetmezliği olan Çocuklarda Büyüme Hormonu Tedavisi Atilla Cayir1, Celalettin Kosan2 1Department of Pediatric Endocrinology, Regional Training and Research Hospital, Erzurum, Turkey 2Department of Pediatric Nephrology, Ataturk University Faculty of Medicine, Erzurum, Turkey Abstract Özet Growth is impaired in a chronic renal failure. Anemia, acidosis, re- Kronik böbrek yetersizliğinde büyüme bozulmaktadır. Anemi, asidoz, duced intake of calories and protein, decreased synthesis of vitamin kalori ve protein alımının azalması, azalmış vitamin D sentezi ve artmış D and increased parathyroid hormone levels, hyperphosphatemia, parathormon düzeyi, hiperfosfatemi, renal osteodistrofi ve büyüme renal osteodystrophy and changes in growth hormone-insulin-like hormonu, insülin benzeri growth faktör ile gonadotropin- gonadal growth factor and the gonadotropin-gonadal axis are implicated in akstaki değişiklikler büyümenin yetersiz olmasından sorumlu tutul- this study. Growth is adversely affected by immunosuppressives and maktadır. Böbrek transplantasyonundan sonra ise immunosupressifler corticosteroids after kidney transplantation. Treating metabolic dis- ve kortikosteroidlerin etkileri ile büyüme olumsuz olarak etkilenmek- orders using the recombinant human growth hormone is an effective tedir. Metabolik bozuklukların düzeltilmesine rağmen büyüme hızı option for patients with inadequate growth rates. yetersiz olan olgularda rekombinant insan büyüme hormonu iyi bir Keywords: Child, chronic renal failure, growth hormone, therapy tedavi seçeneğidir. Anahtar Kelimeler: Çocuk, kronik böbrek yetersizliği, büyüme hor- monu, tedavi Introduction mone takes place with glomerular filtration and breakdown in the proximal tubules. In CRF, a decrease in the rate of glo- Inadequate growth is a widespread problem in children merular filtration leads to impairment of the metabolic clear- with chronic renal failure (CRF). -

A Bioinformatics Model of Human Diseases on the Basis Of
SUPPLEMENTARY MATERIALS A Bioinformatics Model of Human Diseases on the basis of Differentially Expressed Genes (of Domestic versus Wild Animals) That Are Orthologs of Human Genes Associated with Reproductive-Potential Changes Vasiliev1,2 G, Chadaeva2 I, Rasskazov2 D, Ponomarenko2 P, Sharypova2 E, Drachkova2 I, Bogomolov2 A, Savinkova2 L, Ponomarenko2,* M, Kolchanov2 N, Osadchuk2 A, Oshchepkov2 D, Osadchuk2 L 1 Novosibirsk State University, Novosibirsk 630090, Russia; 2 Institute of Cytology and Genetics, Siberian Branch of Russian Academy of Sciences, Novosibirsk 630090, Russia; * Correspondence: [email protected]. Tel.: +7 (383) 363-4963 ext. 1311 (M.P.) Supplementary data on effects of the human gene underexpression or overexpression under this study on the reproductive potential Table S1. Effects of underexpression or overexpression of the human genes under this study on the reproductive potential according to our estimates [1-5]. ↓ ↑ Human Deficit ( ) Excess ( ) # Gene NSNP Effect on reproductive potential [Reference] ♂♀ NSNP Effect on reproductive potential [Reference] ♂♀ 1 increased risks of preeclampsia as one of the most challenging 1 ACKR1 ← increased risk of atherosclerosis and other coronary artery disease [9] ← [3] problems of modern obstetrics [8] 1 within a model of human diseases using Adcyap1-knockout mice, 3 in a model of human health using transgenic mice overexpressing 2 ADCYAP1 ← → [4] decreased fertility [10] [4] Adcyap1 within only pancreatic β-cells, ameliorated diabetes [11] 2 within a model of human diseases -

Recent Advances in Endothelin Research on Cardiovascular and Endocrine Systems
Endocrine Journal 1994, 41(5), 491-507 Review Recent Advances in Endothelin Research on Cardiovascular and Endocrine Systems MITSUHIDE NARUSE, KIYoxo NARUSE, AND HIR0SH1 DEMURA Department of Medicine, Institute of Clinical Endocrinology, TokyoWomen's Medical College, Tokyo162, Japan Introduction Structure of ET and ET Receptor Since the quite exciting discovery in 1988 of The molecular and biochemical aspects of ET endothelin (ET) in the culture medium of aortic en- family peptides and ET receptors have been re- dothelial cells by Yanagisawa and coworkers [1], viewed by Masaki and Yanagisawa [13] and others evidence has accumulated to support its important [14-16]. We here describe the fundamentals. roles in the regulation of blood pressure and body fluid homeostasis and its pathophysiological sig- 1. ET Peptides nificance in various cardiovascular diseases. The discovery of ET and the opponent substances in- The ET molecule consists of 21 amino acid resi- cluding nitric oxide [2] and natriuretic peptides [3] dues with two intramolecular disulfide bonds be- during the last decade was epoch-making in the tween cystein residues at 1 and 15 and 3 and 11, field of "Cardiovascular Endocrinology". respectively. The ring structure and the hydropho- Because of its impressive potency and the long bic amino acid residues at the C-terminus are in- duration of its pressor action [1], the roles of ET in dispensable to its full biological activities. There the regulation of cardiovascular homeostasis have are three distinct isof orms of ET: ET-1, ET-2, and been extensively studied. However, the co-expres- ET-3 [13]. The difference in the amino acid se- sion or close localization of ET and its receptors in quence is seen in the ring structure, while they various tissues other than the vascular vessels sug- share the same sequence in the linear C-terminal gest non-vascular roles of ET [4-6]. -

An Investig Atio N of the Anabolic
AN INVESTIGATION OF THE ANABOLIC ACTIONS OF BIOSYNTHETIC HUMAN GROWTH HORMONE AFTER INJURY BY BURNING A thesis sumitted for the degree of MASTER OF SURGERY in the UNIVERSITY OF LONDON H.J.C.R. Belcher, MB, BS(Lond), FRCS(Eng). The Blond-Mclndoe Centre, Queen Victoria Hospital, Holtye Rd, East Grinstead, SUSSEX. - 1 - ProQuest Number: U053257 All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted. In the unlikely event that the author did not send a com plete manuscript and there are missing pages, these will be noted. Also, if material had to be removed, a note will indicate the deletion. uest ProQuest U053257 Published by ProQuest LLC(2017). Copyright of the Dissertation is held by the Author. All rights reserved. This work is protected against unauthorized copying under Title 17, United States C ode Microform Edition © ProQuest LLC. ProQuest LLC. 789 East Eisenhower Parkway P.O. Box 1346 Ann Arbor, Ml 48106- 1346 To my wife, Georgina - 2 - ABSTRACT Previous clinical trials in normal subjects and post-operative patients have shown that biosynthetic growth hormone preparations increase nitrogen retention. It has been suggested that their administration to injured patients may be beneficial. A clinical trial is presented of twelve adult burned patients of whom six were allocated to receive biosynthetic human growth hormone (somatropin) and six to form a control group. Injury by burning is followed by increases in resting energy expenditure and urinary nitrogen excretion, accompanied by insulin resistance and glucose intolerance. There is a generalised fall in plasma protein concentrations, including the somatomedin, insulin-like growth factor-I. -

Growth Hormone, IGF-1, and Metabolism
www.currentmedicalliterature.com VOLUME 2 | NUMBER 3 | 2009 Growth Growth Hormone, IGF-1, and Metabolism Supported by an unrestricted educational grant from This activity is jointly sponsored by the University of Kentucky College of Medicine and Remedica Medical Education and Publishing. The University of Kentucky is an equal opportunity university. VOLUME 2 | NUMBER 3 | 2009 Growth: Growth Hormone, IGF-1, and Metabolism EDITOR-IN-CHIEF CLINICAL EDITORS NORMAN LAVIN, MD, PhD, FACE, FAAP CRAIG ALTER, MD University of California Los Angeles, The Children’s Hospital of Philadelphia, Los Angeles, CA, USA Philadelphia, PA, USA ADVISORY Board CHARLES BUCHANAN, BSc, MB (Hons), MRCP, FRCPCH DEREK LEROITH, MD, PhD King’s College Hospital, London, UK Mount Sinai School of Medicine, New York, NY, USA HIRALAL MAHESHWARI, MD, PhD Northwestern University Medical School, EDWARD REITER, MD Chicago, IL, USA Tufts University School of Medicine, Springfield, MA, USA RICHARD ROSS, MD, FRCP University of Sheffield, Sheffield, UK Visit CML – Growth online at www.currentmedicalliterature.com The website provides access to new and archived content, a personalized CML Compass search engine, a “Paper of the Month” service, and more! RT107_3_CML_GrowthH_2_3_07.indd 1 22/4/09 10:56:57 CUrrent Medical literatUre JOUrnalS Current Medical Literature journals are designed to solve the problem of information overload for specialist physicians. Each journal is compiled by an editorial team of clinicians from an ongoing review of the international literature, and articles are selected for citation and review on the basis of their relevance to clinical practice. Other titles in the series include the following. For additional information on any of these titles, please contact us at the address below.