Retinal and Choroidal Angiogenesis: a Review of New Targets Thiago Cabral1,2,3, Luiz Guilherme M
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Differential Gene Expression Profile in Endometrioid And
[CANCER RESEARCH 63, 5697–5702, September 15, 2003] Advances in Brief Differential Gene Expression Profile in Endometrioid and Nonendometrioid Endometrial Carcinoma: STK15 Is Frequently Overexpressed and Amplified in Nonendometrioid Carcinomas1 Gema Moreno-Bueno, Carolina Sa´nchez-Este´vez, Rau´l Cassia, Sandra Rodrı´guez-Perales, Ramo´n Dı´az-Uriarte, Orlando Domı´nguez, David Hardisson, Miguel Andujar, Jaime Prat, Xavier Matias-Guiu, Juan C. Cigudosa, and Jose´Palacios2 Laboratory of Breast and Gynaecological Cancer, Molecular Pathology Programme [G. M-B., C. S-E., R. C., J. Pa.] and Biotechnology Programme [S. R-P., R. D-U., O. D., J. C. C.], Centro Nacional de Investigaciones Oncologicas, Madrid; Department of Pathology, Hospital Universitario La Paz, Madrid [D. H.]; Department of Pathology, Hospital Materno Infantil, Las Palmas [M. A.]; Department of Pathology, Hospital Sant Pau y Sant Creu, Barcelona [J. Pr.]; and Department of Pathology, Hospital Arnau de Villanova, Lleida [X. M-G.], Spain Abstract tiated endometrioid carcinomas that usually develop in pre- and perimenopausal women. They are associated with estrogen stimula- Endometrial carcinoma (EC) comprises at least two types of cancer: tion, coexist with, or are preceded by atypical endometrial hyperplasia endometrioid carcinomas (EECs) are estrogen-related tumors, which are and are associated with ER positivity and with K-RAS, PTEN, and frequently euploid and have a good prognosis. Nonendometrioid carcino-  mas (NEECs; serous and clear cell forms) are not estrogen related, are -catenin mutations, and microsatellite instability. Conversely, type II frequently aneuploid, and are clinically aggressive. We used cDNA mi- tumors are NEECs (papillary serous and clear cell carcinomas) that croarrays containing 6386 different genes to analyze gene expression occur in older women. -

DRUGS REQUIRING PRIOR AUTHORIZATION in the MEDICAL BENEFIT Page 1
Effective Date: 08/01/2021 DRUGS REQUIRING PRIOR AUTHORIZATION IN THE MEDICAL BENEFIT Page 1 Therapeutic Category Drug Class Trade Name Generic Name HCPCS Procedure Code HCPCS Procedure Code Description Anti-infectives Antiretrovirals, HIV CABENUVA cabotegravir-rilpivirine C9077 Injection, cabotegravir and rilpivirine, 2mg/3mg Antithrombotic Agents von Willebrand Factor-Directed Antibody CABLIVI caplacizumab-yhdp C9047 Injection, caplacizumab-yhdp, 1 mg Cardiology Antilipemic EVKEEZA evinacumab-dgnb C9079 Injection, evinacumab-dgnb, 5 mg Cardiology Hemostatic Agent BERINERT c1 esterase J0597 Injection, C1 esterase inhibitor (human), Berinert, 10 units Cardiology Hemostatic Agent CINRYZE c1 esterase J0598 Injection, C1 esterase inhibitor (human), Cinryze, 10 units Cardiology Hemostatic Agent FIRAZYR icatibant J1744 Injection, icatibant, 1 mg Cardiology Hemostatic Agent HAEGARDA c1 esterase J0599 Injection, C1 esterase inhibitor (human), (Haegarda), 10 units Cardiology Hemostatic Agent ICATIBANT (generic) icatibant J1744 Injection, icatibant, 1 mg Cardiology Hemostatic Agent KALBITOR ecallantide J1290 Injection, ecallantide, 1 mg Cardiology Hemostatic Agent RUCONEST c1 esterase J0596 Injection, C1 esterase inhibitor (recombinant), Ruconest, 10 units Injection, lanadelumab-flyo, 1 mg (code may be used for Medicare when drug administered under Cardiology Hemostatic Agent TAKHZYRO lanadelumab-flyo J0593 direct supervision of a physician, not for use when drug is self-administered) Cardiology Pulmonary Arterial Hypertension EPOPROSTENOL (generic) -

Supporting Online Material
1 2 3 4 5 6 7 Supplementary Information for 8 9 Fractalkine-induced microglial vasoregulation occurs within the retina and is altered early in diabetic 10 retinopathy 11 12 *Samuel A. Mills, *Andrew I. Jobling, *Michael A. Dixon, Bang V. Bui, Kirstan A. Vessey, Joanna A. Phipps, 13 Ursula Greferath, Gene Venables, Vickie H.Y. Wong, Connie H.Y. Wong, Zheng He, Flora Hui, James C. 14 Young, Josh Tonc, Elena Ivanova, Botir T. Sagdullaev, Erica L. Fletcher 15 * Joint first authors 16 17 Corresponding author: 18 Prof. Erica L. Fletcher. Department of Anatomy & Neuroscience. The University of Melbourne, Grattan St, 19 Parkville 3010, Victoria, Australia. 20 Email: [email protected] ; Tel: +61-3-8344-3218; Fax: +61-3-9347-5219 21 22 This PDF file includes: 23 24 Supplementary text 25 Figures S1 to S10 26 Tables S1 to S7 27 Legends for Movies S1 to S2 28 SI References 29 30 Other supplementary materials for this manuscript include the following: 31 32 Movies S1 to S2 33 34 35 36 1 1 Supplementary Information Text 2 Materials and Methods 3 Microglial process movement on retinal vessels 4 Dark agouti rats were anaesthetized, injected intraperitoneally with rhodamine B (Sigma-Aldrich) to label blood 5 vessels and retinal explants established as described in the main text. Retinal microglia were labelled with Iba-1 6 and imaging performed on an inverted confocal microscope (Leica SP5). Baseline images were taken for 10 7 minutes, followed by the addition of PBS (10 minutes) and then either fractalkine or fractalkine + candesartan 8 (10 minutes) using concentrations outlined in the main text. -

Searching for Novel Peptide Hormones in the Human Genome Olivier Mirabeau
Searching for novel peptide hormones in the human genome Olivier Mirabeau To cite this version: Olivier Mirabeau. Searching for novel peptide hormones in the human genome. Life Sciences [q-bio]. Université Montpellier II - Sciences et Techniques du Languedoc, 2008. English. tel-00340710 HAL Id: tel-00340710 https://tel.archives-ouvertes.fr/tel-00340710 Submitted on 21 Nov 2008 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. UNIVERSITE MONTPELLIER II SCIENCES ET TECHNIQUES DU LANGUEDOC THESE pour obtenir le grade de DOCTEUR DE L'UNIVERSITE MONTPELLIER II Discipline : Biologie Informatique Ecole Doctorale : Sciences chimiques et biologiques pour la santé Formation doctorale : Biologie-Santé Recherche de nouvelles hormones peptidiques codées par le génome humain par Olivier Mirabeau présentée et soutenue publiquement le 30 janvier 2008 JURY M. Hubert Vaudry Rapporteur M. Jean-Philippe Vert Rapporteur Mme Nadia Rosenthal Examinatrice M. Jean Martinez Président M. Olivier Gascuel Directeur M. Cornelius Gross Examinateur Résumé Résumé Cette thèse porte sur la découverte de gènes humains non caractérisés codant pour des précurseurs à hormones peptidiques. Les hormones peptidiques (PH) ont un rôle important dans la plupart des processus physiologiques du corps humain. -

EDNRB Gene Endothelin Receptor Type B
EDNRB gene endothelin receptor type B Normal Function The EDNRB gene provides instructions for making a protein called endothelin receptor type B. This protein is located on the surface of cells and functions as a signaling mechanism, transmitting information from outside the cell to inside the cell. The receptor interacts with proteins called endothelins to regulate several critical biological processes, including the development and function of blood vessels, the production of certain hormones, and the stimulation of cell growth and division. Endothelin 3 (produced from the EDN3 gene) is one of the proteins that interacts with endothelin receptor type B. During early development before birth (embryonic development), endothelin 3 and endothelin receptor type B together play an important role in neural crest cells. These cells migrate from the developing spinal cord to specific regions in the embryo, where they give rise to many different types of cells. In particular, endothelin 3 and endothelin receptor type B are essential for the formation of nerves in the intestine (enteric nerves) and for the production of specialized cells called melanocytes. Melanocytes produce melanin, a pigment that contributes to skin, hair, and eye color. Melanin is also involved in the normal function of the inner ear. Health Conditions Related to Genetic Changes Hirschsprung disease More than 30 mutations in the EDNRB gene have been found to cause Hirschsprung disease, a disorder that causes severe constipation or blockage of the intestine. Although Hirschsprung disease is a feature of another condition called Waardenburg syndrome type IV (described below), EDNRB gene mutations can also cause Hirschsprung disease in people without Waardenburg syndrome. -

BLA 761125 Page 7
BLA 761125 Page 7 HIGHLIGHTS OF PRESCRIBING INFORMATION -----------------------WARNINGS AND PRECAUTIONS---------------------- These highlights do not include all the information needed to use BEOVU Endophthalmitis and retinal detachments may occur following intravitreal safely and effectively. See full prescribing information for BEOVU. injections. Patients should be instructed to report any symptoms suggestive of endophthalmitis or retinal detachment without delay (5.1). BEOVU® (brolucizumab-dbll) injection, for intravitreal injection Increases in intraocular pressure (IOP) have been seen within 30 minutes of Initial U.S. Approval: 2019 an intravitreal injection (5.2). ----------------------------INDICATIONS AND USAGE------------------------- There is a potential risk of arterial thromboembolic events (ATE) following BEOVU is a human vascular endothelial growth factor (VEGF) inhibitor intravitreal use of VEGF inhibitors (5.3). indicated for the treatment of Neovascular (Wet) Age-Related Macular ------------------------------ADVERSE REACTIONS----------------------------- Degeneration (AMD) (1). The most common adverse reactions (≥ 5%) reported in patients receiving ----------------------DOSAGE AND ADMINISTRATION---------------------- BEOVU are vision blurred (10%), cataract (7%), conjunctival hemorrhage BEOVU is administered by intravitreal injection. The recommended dose for (6%), eye pain (5%), and vitreous floaters (5%) (6.1). BEOVU is 6 mg (0.05 mL of 120 mg/mL solution) monthly (approximately To report SUSPECTED ADVERSE REACTIONS, contact Novartis every 25-31 days) for the first three doses, followed by one dose of 6 mg (0.05 Pharmaceuticals Corporation at 1-888-669-6682 or FDA at 1-800-FDA mL) every 8-12 weeks (2). 1088 or www.fda.gov/medwatch. ---------------------DOSAGE FORMS AND STRENGTHS-------------------- See 17 for PATIENT COUNSELING INFORMATION. Injection: 6 mg/0.05 mL solution for intravitreal injection in a single-dose vial (3). -
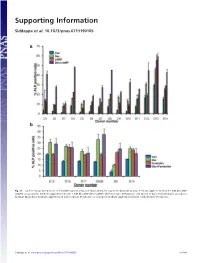
Supporting Information
Supporting Information Siddappa et al. 10.1073/pnas.0711190105 Fig. S1. (a) Percentage ALP-positive cells in hMSCs grown in basic medium (Con), osteogenic medium (Dex), basic medium supplemented with 1 mM db-cAMP (cAMP), or osteogenic medium supplemented with 1 mM db-cAMP (DexϩcAMP). (b) Percentage ALP-positive cells grown in basic medium (Con), osteogenic medium (Dex), basic medium supplemented with forskolin (Forskolin), or osteogenic medium supplemented with forskolin (DexϩForskolin). Siddappa et al. www.pnas.org/cgi/content/short/0711190105 1of10 Fig. S2. hMSCs were grown in basic medium, basic medium supplemented with 1 mM db-cAMP (cAMP), osteogenic medium (Dex), or osteogenic medium supplemented with 1 mM db-cAMP (DexϩcAMP). Expression was analyzed by qPCR and is expressed as fold induction compared with cells grown in basic medium. The data were analyzed by using two-way ANOVA, and statistical significance is indicated compared with cells grown in basic medium. *, P Ͻ 0.05. Siddappa et al. www.pnas.org/cgi/content/short/0711190105 2of10 Fig. S3. (a) Methylene blue staining of hMSC-seeded scaffolds grown in basic medium (Con) or basic medium supplemented with 1 mM db-cAMP (cAMP) for 4 days. Note the less intensely stained db-cAMP-treated construct, indicating reduced cell numbers. (b) Quantitative Alamar blue assay for cell number analysis. The data were analyzed by using one-way ANOVA followed by Dunnet’s multiple-comparison test. Statistical significance is indicated compared with cells grown in basic medium (Con). *, P Ͻ 0.05 Siddappa et al. www.pnas.org/cgi/content/short/0711190105 3of10 Fig. -

Regeneron Needs a New Plan B for Eylea
November 27, 2017 Regeneron needs a new plan B for Eylea Madeleine Armstrong Regeneron’s efforts to extend the lifespan of its blockbuster Eylea franchise via combinations have fallen flat again, raising the question of whether it can find anything that can best Eylea alone. With competition on the horizon from Novartis’s rival wet age-related macular degeneration (AMD) project, brolucizumab, Eylea sales are forecast to flatten after 2020 – something that Regeneron cannot now counter with combos (see table below). Regeneron’s stock was down 3% in premarket trading this morning, but the shares opened down just 1%. Investors might have been reassured by the fact that full data from the Hawk and Harrier studies of brolucizumab, released earlier this month, were not the smash hit that Novartis had hoped for. This could allow Eylea to keep its dominant position in the wet age-related macular degeneration (AMD) market for some time to come. Top wet AMD drugs in 2022 Annual indication sales ($m) Product Company Status 2016 2018e 2020e 2022e Eylea Regeneron/Bayer/Santen Pharmaceutical Marketed 3,584 3,875 4,073 3,942 Lucentis Novartis/Roche Marketed 2,354 2,271 1,924 1,460 Brolucizumab Novartis Phase III - - 319 937 Source: EvaluatePharma. And if positive, the phase III Panorama study of Eylea monotherapy in diabetic retinopathy, due to report in the first half of next year, could open up another avenue for growth for the company’s most valuable product. But even the most optimistic Regeneron bulls will have to admit that Eylea’s sales are likely to be eroded by the market entry of brolucizumab, expected in 2019 or 2020. -
![A Genomic Atlas of Human Adrenal and Gonad Development [Version 2; Referees: 4 Approved] Ignacio Del Valle1, Federica Buonocore1, Andrew J](https://docslib.b-cdn.net/cover/1314/a-genomic-atlas-of-human-adrenal-and-gonad-development-version-2-referees-4-approved-ignacio-del-valle1-federica-buonocore1-andrew-j-711314.webp)
A Genomic Atlas of Human Adrenal and Gonad Development [Version 2; Referees: 4 Approved] Ignacio Del Valle1, Federica Buonocore1, Andrew J
Wellcome Open Research 2017, 2:25 Last updated: 08 NOV 2017 RESEARCH ARTICLE A genomic atlas of human adrenal and gonad development [version 2; referees: 4 approved] Ignacio del Valle1, Federica Buonocore1, Andrew J. Duncan1, Lin Lin1, Martino Barenco2, Rahul Parnaik1, Sonia Shah3,4, Mike Hubank5, Dianne Gerrelli2, John C. Achermann 1 1Genetics and Genomic Medicine, UCL Great Ormond Street Institute of Child Health, London, UK 2Developmental Biology and Cancer, UCL Great Ormond Street Institute of Child Health, London, UK 3Institute for Molecular Bioscience, University of Queensland, Brisbane, Australia 4Institute of Cardiovascular Science, University College London, London, UK 5The Centre for Molecular Pathology, Royal Marsden Hospital, Sutton, UK v2 First published: 07 Apr 2017, 2:25 (doi: 10.12688/wellcomeopenres.11253.1) Open Peer Review Latest published: 23 Oct 2017, 2:25 (doi: 10.12688/wellcomeopenres.11253.2) Referee Status: Abstract Background: In humans, the adrenal glands and gonads undergo distinct biological events between 6-10 weeks post conception (wpc), such as testis Invited Referees determination, the onset of steroidogenesis and primordial germ cell 1 2 3 4 development. However, relatively little is currently known about the genetic mechanisms underlying these processes. We therefore aimed to generate a detailed genomic atlas of adrenal and gonad development across these critical version 2 report report stages of human embryonic and fetal development. published Methods: RNA was extracted from 53 tissue samples between 6-10 wpc 23 Oct 2017 (adrenal, testis, ovary and control). Affymetrix array analysis was performed and differential gene expression was analysed using Bioconductor. A version 1 mathematical model was constructed to investigate time-series changes across published report report report report 07 Apr 2017 the dataset. -

Table of Contents
Therapeutic systems for Insulin-like growth factor-I Dissertation zur Erlangung des naturwissenschaftlichen Doktorgrades der Julius-Maximilians-Universität Würzburg vorgelegt von Isabel Schultz aus Dahn Würzburg 2015 Eingereicht bei der Fakultät für Chemie und Pharmazie am Gutachter der schriftlichen Arbeit 1. Gutachter: 2. Gutachter: Prüfer des öffentlichen Promotionskolloquiums 1. Prüfer: 2. Prüfer: 3. Prüfer: Datum des öffentlichen Promotionskolloquiums Doktorurkunde ausgehändigt am TABLE OF CONTENTS TABLE OF CONTENTS SUMMARY ............................................................................... 1 ZUSAMMEMFASSUNG .......................................................... 5 CHAPTER I ............................................................................... 9 DRUG DELIVERY OF INSULIN-LIKE GROWTH FACTOR I CHAPTER II ............................................................................ 45 INSULIN-LIKE GROWTH FACTOR-I AEROSOL FORMULATIONS FOR PULMONARY DELIVERY CHAPTER III ........................................................................... 73 PULMONARY INSULIN-LIKE GROWTH FACTOR I DELIVERY FROM TREHALOSE AND SILK-FIBROIN MICROPARTICLES CHAPTER IV ......................................................................... 113 EXPRESSION OF IGF-I MUTANTS CONCLUSION AND OUTLOOK ......................................... 147 DOCUMENTATION OF AUTHORSHIP ............................. 159 CURRICULUM VITAE ......................................................... 163 ACKNOWLEDGMENTS ..................................................... -

July 1, 2020 RE: Physician Administered Drugs (J Codes)
July 1, 2020 RE: Physician Administered Drugs (J Codes) Included in Prior Authorization Matrix for Molina Healthcare of New York, Inc. Dear Participating Provider: Molina Healthcare of New York, Inc. requires prior authorization for these HCPCS codes for all participating providers. Prior authorization is required before services are rendered for the codes listed. Prior authorization requests should include the most up to date patient information including office notes, consultations, labs, scans, previous treatments tried, and any other patient specific information to support the request. Please fax a completed prior authorization form along with all supporting clinical documentation to fax number: 1-844- 823-5479. Our prior authorization form and most up to date formulary can be found on our website: MolinaHealthcare.com. C9061 INJECTION, TEPROTUMUMAB-TRBW, 10 mg C9063 INJECTION, EPTINEZUMAB-JJMR, 1 mg C9122 MOMETASONE FUROATE SINUS IMPLANT, 10 micrograms (sinuva) J0122 INJECTION, ERAVACYCLINE, 1 mg J0129 Injection, abatacept 10 mg J0178 Injection, aflibercept 1 mg J0179 INJECTION, BROLUCIZUMAB-DBLL, 1MG J0185 Injection, aprepitant 1 mg J0223 INJECTION, GIVOSIRAN, 0.5 mg J0285 INJECTION, AMPHOTERICIN B, 50 mg J0490 Injection, belimumab 10 mg J0517 Injection, benralizumab 1 mg J0567 Injection, cerliponase alfa 1 mg J0584 Injection, burosumab-twza 1 mg J0585 Injection, onabotulinumtoxina, 1 unit J0587 Injection, rimabotulinumtoxinb 100 units J0599 Injection, c-1 esterase inhibitor 10 units J0641 Injection, levoleucovorin, not otherwise -

Thymic Mesenchymal Cells Have a Distinct Transcriptomic Profile
Thymic Mesenchymal Cells Have a Distinct Transcriptomic Profile Julien Patenaude and Claude Perreault This information is current as J Immunol 2016; 196:4760-4770; Prepublished online 29 of October 1, 2021. April 2016; doi: 10.4049/jimmunol.1502499 http://www.jimmunol.org/content/196/11/4760 Downloaded from Supplementary http://www.jimmunol.org/content/suppl/2016/04/29/jimmunol.150249 Material 9.DCSupplemental References This article cites 65 articles, 18 of which you can access for free at: http://www.jimmunol.org/content/196/11/4760.full#ref-list-1 http://www.jimmunol.org/ Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication by guest on October 1, 2021 *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2016 by The American Association of Immunologists, Inc. All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. The Journal of Immunology Thymic Mesenchymal Cells Have a Distinct Transcriptomic Profile Julien Patenaude and Claude Perreault In order to understand the role of mesenchymal cells (MCs) in the adult thymus, we performed whole transcriptome analyses of primary thymic, bone, and skin MCs.