Distribution and Abundance of Panulirus Spp
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lobsters-Identification, World Distribution, and U.S. Trade
Lobsters-Identification, World Distribution, and U.S. Trade AUSTIN B. WILLIAMS Introduction tons to pounds to conform with US. tinents and islands, shoal platforms, and fishery statistics). This total includes certain seamounts (Fig. 1 and 2). More Lobsters are valued throughout the clawed lobsters, spiny and flat lobsters, over, the world distribution of these world as prime seafood items wherever and squat lobsters or langostinos (Tables animals can also be divided rougWy into they are caught, sold, or consumed. 1 and 2). temperate, subtropical, and tropical Basically, three kinds are marketed for Fisheries for these animals are de temperature zones. From such partition food, the clawed lobsters (superfamily cidedly concentrated in certain areas of ing, the following facts regarding lob Nephropoidea), the squat lobsters the world because of species distribu ster fisheries emerge. (family Galatheidae), and the spiny or tion, and this can be recognized by Clawed lobster fisheries (superfamily nonclawed lobsters (superfamily noting regional and species catches. The Nephropoidea) are concentrated in the Palinuroidea) . Food and Agriculture Organization of temperate North Atlantic region, al The US. market in clawed lobsters is the United Nations (FAO) has divided though there is minor fishing for them dominated by whole living American the world into 27 major fishing areas for in cooler waters at the edge of the con lobsters, Homarus americanus, caught the purpose of reporting fishery statis tinental platform in the Gul f of Mexico, off the northeastern United States and tics. Nineteen of these are marine fish Caribbean Sea (Roe, 1966), western southeastern Canada, but certain ing areas, but lobster distribution is South Atlantic along the coast of Brazil, smaller species of clawed lobsters from restricted to only 14 of them, i.e. -

First Record and Description of Panulirus Pascuensis (Reed, 1954) First Stage Phyllosoma in the Plankton of Rapa Nui
First record and description of Panulirus pascuensis (Reed, 1954) first stage phyllosoma in the plankton of Rapa Nui By Meerhoff Erika*, Mujica Armando, García Michel, and Nava María Luisa Abstract Among the most striking crustaceans from Rapa Nui Island (27° S, 109°22’ W) is the endemic lobster Panulirus pascuensis, commonly known as the spiny lobster. This species is also present in Pitcairn Island and the Salas y Gomez ridge. The larvae of this species pass through different phyllosoma stages until metamorphosis, in which they molt to puerulus, a transitional stage adapted to benthic life. This species is an important fishery resource for the inhabitants of Rapa Nui. However, there are several gaps in the biological knowledge of this species, including its ontogeny. We sampled zooplankton to obtain first stage P. pascuensis phyllosoma during three oceanographic campaigns around Rapa Nui (April 2015, September 2015 and March 2016), individuals were encountered between the surface and 200 m depth with an abundance of 1.3 indiv/1000 m3. These individuals, along with laboratory hatched phyllosoma, allowed us to describe the morphology of this larval stage. The P. pascuensis stage I phyllosoma were observed in fall, suggesting that the larval development would be synchronized with the productivity cycle in the region of Rapa Nui, where maximum chlorophyll concentration is observed during the austral winter. *Corresponding Author E-mail: [email protected] Pacific Science, vol. 71, no. 2 December 9, 2016 (Early view) Introduction The island of Rapa Nui (also known as Easter Island) is part of the Marine and Coastal Protected Area called Coral Nui Nui, Motu Tautara and Hanga Oteo submarine Parks (Sierralta et al. -

Guide to the Lobsters and Lobster-Like Animals of Florida, the Gulf Of
Sea Grant Field Guide Series Pl !t p! g jQ4'4JtII Guideto the Lobsters and I obster-like Animals of Florida, the Gulf of Mexico and the CaribbeanRegion Lee Opresko, Dennis Opresko Ronald Thomas and Gilbert Voss Edited and Illustrat.ed by Frederick IH. Bayer University of Ifftami Sea Grant Program hIDAASea Grant No. 04-3-t58-27! Miami, Florl da I973 Foreword The University of Miami Sea Grant Field Guide Series is published to make available to the commercial and sports fishermen, the gen- The University of Miami's Sea Grant Programis a part of the Nationa} eral public, and fisheries and conservation personnel easily usable, SeaGrant Program, which is administered by the National Oceanicand non-technical, well-illustrated guides for the identification of AtmosphericAdministration cf the U.S. Departmentof Conmerce. the marine life of the area. Every means has been used to avoid technical terms where possible. When these must be used to avoid confusion, they are carefully explained and often illustrated. Glossaries are included when thought necessary. But the guides go further than just identification. Where such knowledge is available, information is given on geographical dis- tribution, depth distribution, abundance, time of spawning, present utilization, means of harvesting and mariculture methods, besides other useful information when known. Price: $3.00 The format is uniform in the series for greater ease of use. Actu- al photographs are used where possible but when greater clarity is required, drawings are used. In general we have attempted to illus- trate each species but in cases where two or more species are very similar, this is noted, a single illustration is used, and dis- tinguishing characters are given in the text. -

1 Modeling Spiny Lobster Larval Dispersion in the Tropical Atlantic
Modeling spiny lobster larval dispersion in the tropical Atlantic using satellite data. 1 Camila Aguirre Góes João Antônio Lorenzzetti 1 Douglas F. Marcolino Gherardi 1 Jorge Eduardo Lins Oliveira 2 1 Instituto Nacional de Pesquisas Espaciais - INPE Caixa Postal 515 - 12245-970 - São José dos Campos - SP, Brasil {camila, loren, douglas}@dsr.inpe.br ² Universidade Federal do Rio Grande do Norte - UFRN/DOL Av. Praia de Mãe Luíza s/n; Via Costeira, Cep: 59.014-100 - Natal - RN, Brasil [email protected] Abstract. Spiny lobsters (Palinuridae) have a relatively long planktonic larval phase lasting around one year. Therefore, the mean ocean currents are expected to have a potential for transporting lobster larvae over long distances away from the original spawning areas. We have investigated larval dispersion across tropical Atlantic (20ºN-15ºS; 15ºE-45ºW), using an advection-diffusion model in an attempt to determine the connectivity among different adult stocks. The model updates the position of each larva everyday, along 365 days using the surface geostrophic velocity fields derived from altimeter satellite. Results of simulations indicate that the larval stocks from the African coast do not spread far from the spawning areas. Simulations also indicate that the Brazilian adult stocks could be maintained with larvae released from oceanic islands such as São Pedro and São Paulo Archipelago, Atol das Rocas and Fernando de Noronha. Finally, it is possible that middle South-Atlantic islands (i. e. Ascension Island), could act as stepping-stones between the African and the South-American spiny lobster stocks. Key-words : Larvae dispersion, altimeter satellite, Palinuridae, phyllosoma , advection-diffusion model. -

5. Index of Scientific and Vernacular Names
click for previous page 277 5. INDEX OF SCIENTIFIC AND VERNACULAR NAMES A Abricanto 60 antarcticus, Parribacus 209 Acanthacaris 26 antarcticus, Scyllarus 209 Acanthacaris caeca 26 antipodarum, Arctides 175 Acanthacaris opipara 28 aoteanus, Scyllarus 216 Acanthacaris tenuimana 28 Arabian whip lobster 164 acanthura, Nephropsis 35 ARAEOSTERNIDAE 166 acuelata, Nephropsis 36 Araeosternus 168 acuelatus, Nephropsis 36 Araeosternus wieneckii 170 Acutigebia 232 Arafura lobster 67 adriaticus, Palaemon 119 arafurensis, Metanephrops 67 adriaticus, Palinurus 119 arafurensis, Nephrops 67 aequinoctialis, Scyllarides 183 Aragosta 120 Aesop slipper lobster 189 Aragosta bianca 122 aesopius, Scyllarus 216 Aragosta mauritanica 122 affinis, Callianassa 242 Aragosta mediterranea 120 African lobster 75 Arctides 173 African spear lobster 112 Arctides antipodarum 175 africana, Gebia 233 Arctides guineensis 176 africana, Upogebia 233 Arctides regalis 177 Afrikanische Languste 100 ARCTIDINAE 173 Agassiz’s lobsterette 38 Arctus 216 agassizii, Nephropsis 37 Arctus americanus 216 Agusta 120 arctus, Arctus 218 Akamaru 212 Arctus arctus 218 Akaza 74 arctus, Astacus 218 Akaza-ebi 74 Arctus bicuspidatus 216 Aligusta 120 arctus, Cancer 217 Allpap 210 Arctus crenatus 216 alticrenatus, Ibacus 200 Arctus crenulatus 218 alticrenatus septemdentatus, Ibacus 200 Arctus delfini 216 amabilis, Scyllarus 216 Arctus depressus 216 American blunthorn lobster 125 Arctus gibberosus 217 American lobster 58 Arctus immaturus 224 americanus, Arctus 216 arctus lutea, Scyllarus 218 americanus, -

Natural Diet of the Spiny Lobster, Panulirus Echinatus Smith, 1869
Natural diet of the spiny lobster, Panulirus echinatus Smith, 1869 (Crustacea: Decapoda: Palinuridae), from São Pedro and São Paulo Archipelago, Brazil Góes, CA.a* and Lins-Oliveira, JE.b aDivisão de Sensoriamento Remoto, Instituto Nacional de Pesquisas Espaciais – INPE, Av. dos Astronautas, nº 1758, Jardim da Granja, CEP 12227-010, São José dos Campos, SP, Brazil bLaboratório de Biologia Pesqueira, Departamento de Oceanografia e Limnologia, Universidade Federal do Rio Grande do Norte – UFRN, Av. Praia de Mãe Luíza s/n, Via Costeira, CEP 59014-100, Natal, RN, Brazil *e-mail: [email protected] Received March 26, 2007 – Accepted June 1, 2007 – Distributed February 28, 2009 (With 3 figures) Abstract The natural diet of the spiny lobster (Panulirus echinatus) from the São Pedro and São Paulo Archipelago was de- termined by stomach contents analysis of sixty-eight adult lobsters collected during October 2002, March, July and August 2003. Food items were grouped by gross taxa representing 11 food categories. Analysis included a qualita- tive (Frequency of Occurrence of item i - FOi) and a quantitative method (Volume of item i - Vi). A Feed Index (FI = FOi x Vi / 100) proposed by Lauzanne (1975) was also used to indicate the importance of each food category (>50: dominant, 25-50: essential, 10-25: unimportant, <10: secondary). The most important food category determined by % FOi and % Vi, was fish, followed by crustaceans, green algae, calcareous algae, and rocks. The remaining food categories contributed to less than 30% of FOi and 10% of Vi. Although the Feed Index revealed no dominant food category in the diet of P. -

Ascension Island BAP: Panulirus Echinatus SUMMARY Photo: J. Brown
Ascension Island BAP: Panulirus echinatus Photo: J. Brown SUMMARY Taxonomy: Kingdom: Animalia; Phylum: Arthropoda; Class: Malacostraca; Order: Decapoda; Family: Palinuridae; Species: Panulirus echinatus Nativeness: Native, breeding Description: Medium-sized, nocturnally active marine invertebrate with carapace length of up to 19cm (males) and 15cm (females). Found in rocky habitats where crevices and other refuges are present, down to a depth of around 30m. Adults are predominantly opportunistic, feeding on a variety of marine food sources, including scavenged material. IUCN Red List status: Least Concern Local trend: Unknown Threats: Principle threat to this species harvesting in the recreational inshore fishery (at Ascension Island) and overexploitation by commercial and artisanal fisheries in many other areas of this species’ range Citation: Ascension Island Government (2015) Spiny lobster species action plan. In: The Ascension Island Biodiversity Action Plan. Ascension Island Government Conservation Department, Georgetown, Ascension Island. Ascension Island BAP: Panulirus echinatus Distribution Global Occurs in the tropical Western Atlantic and around islands of the Central Atlantic, including Ascension Island, St. Helena and Cape Verde [1,6,10,11]. Local Spiny lobster are found around the coast of Ascension Island, where there is rocky habitat, but are often seen more frequently on sublittoral lava flows such as those to the northwest, northeast and southwest of the island. However, distribution at a local scale is poorly understood, both due to their nocturnal nature and also a poor reporting rate from marine users, often due to concerns over “poaching” if locations are revealed. Suitable habitat is potentially distributed around the island, with the exception of several sandy bays but gaps in substrate mapping exist. -
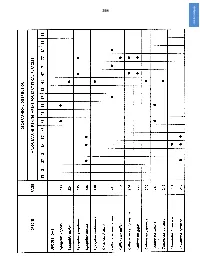
Click for Next Page Click for Previous Page 265
264 click for next page click for previous page 265 4. BIBLIOGRAPHY Albert, F., 1898. La langosta de Juan Fernandez i la posibilidad de su propagación en la costa Chilena. Revista Chilena Historia natural, 2:5-11, 17-23,29-31, 1 tab Alcock, A., 1901. A descriptive catalogue of the Indian deep-Sea Crustacea Decapoda Macrura and Anomala in the Indian Museum. Being a revised account of the deep-sea species collected by the Royal Indian Marine Survey Ship Investigator: 1-286, i-iv, pls 1-3 Alcock, A. & A.R.S. Anderson, 1896. Illustrations of the Zoology of the Royal Indian Marine Surveying Steamer Investigator, under the command of Commander CF. Oldham, R.N. Crustacea (4):pls 1-6-27 Alcock, A. & A.F. McArdle, 1902. Illustrations of the Zoology of the Royal Indian Marine Survey Ship Investigator, under the command of Captain T.H. Heming, R.N. Crustacea, (10), pls 56-67 Allsopp, W.H.L., 1968. Report to the government of British Honduras (Belize) on investigations into marine fishery management, research and development policy for Spiny Lobster fisheries. Report U.N. Development Program FAO, TA 2481 :i-xii, 1-86, figs 1-15 Arana Espina, P. & C.A. Melo Urrutia, 1973. Pesca comercial’de Jasus frontalis en las Islas Robinson Crusoe y Santa Clara. (1971-1972). La Langosta de Juan Fernández II. Investigaciones Marinas, Valparaiso, 4(5):135-152, figs 1-5. For no. I see next item, for III see Pizarro et al., 1974, for IV see Pavez Carrera et al., 1974 Arana Espina, P. -

Downloaded from Genbank for All Australian Palinurid And
ResearchOnline@JCU This is the author-created version of the following work: Woodings, Laura N., Murphy, Nicholas P., Jeffs, Andrew, Suthers, Iain M., Liggins, Geoffrey, and Strugnell, Jan M. (2019) Distribution of Palinuridae and Scyllaridae phyllosoma larvae within the East Australian Current: a climate change hot spot. Marine and Freshwater Research, 70 pp. 1020-1033. Access to this file is available from: https://researchonline.jcu.edu.au/61097/ Journal compilation © CSIRO 2019. The Accepted Manuscript version of this paper is available Open Access from ResearchOnline@JCU by permission of the publisher. Please refer to the original source for the final version of this work: https://doi.org/10.1071/MF18331 Title: Distribution of Palinuridae and Scyllaridae phyllosoma larvae within the East Australian Current: a climate change hot-spot Authors: Laura N. WoodingsA,G, Nick P. MurphyA, Andrew JeffsB, Iain M. SuthersC, Geoffrey W. LigginsD, Bridget S. GreenE, Jan M. StrugnellA,F. ADepartment of Ecology, Environment and Evolution, School of Life Sciences, La Trobe University, Bundoora, Vic. 3086, Australia. BSchool of Biological Sciences and Institute of Marine Science, University of Auckland, Auckland 1142, New Zealand. CSchool of Biological, Earth & Environmental Sciences, University of New South Wales, Sydney, NSW 2052, Australia. DNSW Department of Primary Industries, Sydney Institute of Marine Science, Mosman NSW 2088, Australia EInstitute for Marine and Antarctic Studies, University of Tasmania, Hobart, Tas. 7001, Australia FCentre for Sustainable Tropical Fisheries and Aquaculture and College of Science and Engineering, James Cook University, Townsville, Qld 4811, Australia. GCorresponding author: Email: [email protected] Abstract Many marine species are predicted to shift their ranges poleward due to rising ocean temperatures driven by climate change. -

Feeding Habits of the Scalloped Spiny Lobstrer, Panulirus Homarus (Linnaeus, 1758) (Decapoda: Palinuridae) from the South East Coast Of
www.trjfas.org ISSN 1303-2712 Turkish Journal of Fisheries and Aquatic Sciences 11: 45-54 (2011) DOI: 10.4194/trjfas.2011.0107 Feeding Habits of the Scalloped Spiny Lobstrer, Panulirus homarus (Linnaeus, 1758) (Decapoda: Palinuridae) from the South East Coast of Iran Nassrin Mashaii1,*, Farhad Rajabipour1, Arash Shakouri2 1 Iranian Fisheries Research Organization, Brackish Water Fisheries Research Station, Bafq, Po.Box 89715/1123, Yazd, Iran. 2 Chabahar University of Maritime, Dept. of Marine Biology, Daneshgah Blvd., Chabahar, Iran. * Corresponding Author: Tel.: +98.352 422992; Fax: +98.352 422993; Received 01 June 2009 E-mail: [email protected] Accepted 01 October 2010 Abstract Certain ecological aspects of the feeding habits of 260 scalloped lobsters Panulirus homarus collected during monthly dives off the southeast coast of Iran were considered. Parameters under consideration included: water temperature, salinity, turbidity, pH, dissolved oxygen and macrobenthose of the lobsters habitat. The mean number of empty stomachs varied from 25% to 62%. Statistical analysis revealed no monthly or seasonal significant difference in the frequency of non-empty stomachs (P>0.05). 1 to 6 prey items were distinguishable in each non-empty stomach. Monthly mean of point counts of prey obtained was from 8.76±4.18 to 154.95±73.62, without any significant difference by Tukeys’ HSD test. Bivalves often had 2 the maximum amounts of Fi and Pi amongst different prey items. Pearson χ test did not show any significant difference between frequency of occurrence of each prey item with different length classes, sex, moulting condition and presence of eggs in females (P>0.05). -

Rapporto Finale
“Monitoraggio delle risorse ittiche e della pesca: indagine sugli spostamenti di specie marine di interesse commerciale e conservazionistico” Giacalone V.M. 1, Zenone A. 2, D’Anna G. 2, Buffa G. 1, Patti C. 1, Di Stefano G. 2, Pipitone C. 2, Badalamenti F. 2, Gristina M. 3 1 CNR-IAMC Capo Granitola, via del mare 3 – 91021 – Torretta Granitola (Fraz. Campobello di Mazara, TP), Italia 2 CNR-IAMC Castellammare del Golfo, via D. da Verrazzano 17 – 91014 – Castellammare del Golfo (TP), Italia 3 CNR-IAMC Mazara del Vallo, via L. Vaccara 61 – 91026 – Castellammare del Golfo (TP), Italia Il presente rapporto tecnico documenta le attività svolte nel periodo dal 19/03/2015 (firma atto d’obbligo) al 19/06/2016 (scadenza progetto a seguito di proroga concessa con decreto n°59 del 04/02/2016). Il cronoprogramma realmente seguito ha incluso le seguenti attività: Mese Attività 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 Raccolta Bibliografica Caratterizzazione area di studio Set-up sperimentale (range test) Reperimento esemplari Telemetria Analisi dati Elaborazione report Ridefinizione piano di protezione Nei paragrafi successivi, verranno descritte in dettaglio le varie attività unitamente ai risultati ottenuti in ciascuna fase. RACCOLTA BIBLIOGRAFICA È stato effettuato un aggiornamento della bibliografia in possesso dal CNR-IAMC degli articoli pubblicati su riviste internazionali o capitoli di libri, grazie al motore di ricerca “ISI-web of Knownledge” della Thomson Reuters. Le parole-chiave, e relative combinazioni, utilizzate per la ricerca sono state: - Spiny lobsters - Acoustic telemetry - Palinurus elephas - Marine Protected Areas - Movement pattern - Activity pattern I riferimenti bibliografici vanno dal 1956 ai giorni nostri e, laddove possibile, sono stati reperiti gli articoli in formato pdf. -
Harvest Slot Limits and Marine Protected Areas for the Management of the Caribbean Spiny Lobster
Old Dominion University ODU Digital Commons Biological Sciences Theses & Dissertations Biological Sciences Summer 2018 The Importance of Keeping the Big Ones: Harvest Slot Limits and Marine Protected Areas for the Management of the Caribbean Spiny Lobster Gayathiri Gnanalingam Old Dominion University, [email protected] Follow this and additional works at: https://digitalcommons.odu.edu/biology_etds Part of the Aquaculture and Fisheries Commons, Ecology and Evolutionary Biology Commons, and the Natural Resources Management and Policy Commons Recommended Citation Gnanalingam, Gayathiri. "The Importance of Keeping the Big Ones: Harvest Slot Limits and Marine Protected Areas for the Management of the Caribbean Spiny Lobster" (2018). Doctor of Philosophy (PhD), Dissertation, Biological Sciences, Old Dominion University, DOI: 10.25777/hhrk-9p92 https://digitalcommons.odu.edu/biology_etds/31 This Dissertation is brought to you for free and open access by the Biological Sciences at ODU Digital Commons. It has been accepted for inclusion in Biological Sciences Theses & Dissertations by an authorized administrator of ODU Digital Commons. For more information, please contact [email protected]. THE IMPORTANCE OF KEEPING THE BIG ONES: HARVEST SLOT LIMITS AND MARINE PROTECTED AREAS FOR THE MANAGEMENT OF THE CARIBBEAN SPINY LOBSTER by Gayathiri Gnanalingam BSc August 2008, Otago University, New Zealand LLB August 2008, Otago University, New Zealand MSc December 2013, Otago University, New Zealand A Dissertation Submitted to the Faculty of Old Dominion University in Partial Fulfillment of the Requirements for the Degree of DOCTOR OF PHILOSOPHY ECOLOGICAL SCIENCES OLD DOMINON UNIVERSITY August 2018 Approved by: Mark J. Butler, IV (Director) Holly Gaff (Member) Alison MacDiarmid (Member) ABSTRACT THE IMPORTANCE OF KEEPING THE BIG ONES: HARVEST SLOT LIMITS AND MARINE PROTECTED AREAS FOR THE MANAGEMENT OF THE CARIBBEAN SPINY LOBSTER Gayathiri Gnanalingam Old Dominion University, 2018 Director: Dr.