Penicillatus
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lobsters-Identification, World Distribution, and U.S. Trade
Lobsters-Identification, World Distribution, and U.S. Trade AUSTIN B. WILLIAMS Introduction tons to pounds to conform with US. tinents and islands, shoal platforms, and fishery statistics). This total includes certain seamounts (Fig. 1 and 2). More Lobsters are valued throughout the clawed lobsters, spiny and flat lobsters, over, the world distribution of these world as prime seafood items wherever and squat lobsters or langostinos (Tables animals can also be divided rougWy into they are caught, sold, or consumed. 1 and 2). temperate, subtropical, and tropical Basically, three kinds are marketed for Fisheries for these animals are de temperature zones. From such partition food, the clawed lobsters (superfamily cidedly concentrated in certain areas of ing, the following facts regarding lob Nephropoidea), the squat lobsters the world because of species distribu ster fisheries emerge. (family Galatheidae), and the spiny or tion, and this can be recognized by Clawed lobster fisheries (superfamily nonclawed lobsters (superfamily noting regional and species catches. The Nephropoidea) are concentrated in the Palinuroidea) . Food and Agriculture Organization of temperate North Atlantic region, al The US. market in clawed lobsters is the United Nations (FAO) has divided though there is minor fishing for them dominated by whole living American the world into 27 major fishing areas for in cooler waters at the edge of the con lobsters, Homarus americanus, caught the purpose of reporting fishery statis tinental platform in the Gul f of Mexico, off the northeastern United States and tics. Nineteen of these are marine fish Caribbean Sea (Roe, 1966), western southeastern Canada, but certain ing areas, but lobster distribution is South Atlantic along the coast of Brazil, smaller species of clawed lobsters from restricted to only 14 of them, i.e. -
Lobsters LOBSTERS§
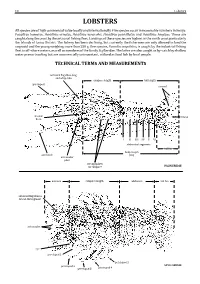
18 Lobsters LOBSTERS§ All species are of high commercial value locally and internationally. Five species occur in reasonable numbers in Kenya: Panulirus homarus, Panulirus ornatus, Panulirus versicolor, Panulirus penicillatus and Panulirus longipes. These are caughtungravid along and the the coast young by weighing the artisanal more fishing than 250 fleet. g. Landings One species, of these Puerulus species angulatus are highest in the north coast particularly the Islands of Lamu District. The fishery has been declining,Scyllaridae. but currently The latter the fishermen are also caught are only as by–catch allowed toby landshallow the , is caught by the industrial fishing fleet in off–shore waters, as well as members of the family water prawn trawling but areTECHNICAL commercially unimportant, TERMS AND utilized MEASUREMENTS as food fish by local people. and whip–like antennal flagellum long carapace length tail length pereiopod uropod frontal telson horn III III IV VIV abdominal segments tail fan body length antennule (BL) antennular plate strong spines on carapace PALINURIDAE antenna carapace length abdomen tail fan antennal flagellum a broad, flat segment antennules eye pereiopod 1 pereiopod 5 pereiopod 2 SCYLLARIDAE pereiopod 3 pereiopod 4 Guide to Families 19 GUIDE TO FAMILIES NEPHROPIDAE Page 20 True lobsters § To about 15 cm. Marine, mainly deep waters on soft included in the Guide to Species. 1st pair of substrates. Three species of interest to fisheriespereiopods are large 3rd pair of pereiopods with chela PALINURIDAE Page 21 Antennal Spiny lobsters § To about 50 cm. Marine, mostly shallow waters on flagellum coral and sand stone reefs, some species on soft included in the Guide to Species. -

First Record and Description of Panulirus Pascuensis (Reed, 1954) First Stage Phyllosoma in the Plankton of Rapa Nui
First record and description of Panulirus pascuensis (Reed, 1954) first stage phyllosoma in the plankton of Rapa Nui By Meerhoff Erika*, Mujica Armando, García Michel, and Nava María Luisa Abstract Among the most striking crustaceans from Rapa Nui Island (27° S, 109°22’ W) is the endemic lobster Panulirus pascuensis, commonly known as the spiny lobster. This species is also present in Pitcairn Island and the Salas y Gomez ridge. The larvae of this species pass through different phyllosoma stages until metamorphosis, in which they molt to puerulus, a transitional stage adapted to benthic life. This species is an important fishery resource for the inhabitants of Rapa Nui. However, there are several gaps in the biological knowledge of this species, including its ontogeny. We sampled zooplankton to obtain first stage P. pascuensis phyllosoma during three oceanographic campaigns around Rapa Nui (April 2015, September 2015 and March 2016), individuals were encountered between the surface and 200 m depth with an abundance of 1.3 indiv/1000 m3. These individuals, along with laboratory hatched phyllosoma, allowed us to describe the morphology of this larval stage. The P. pascuensis stage I phyllosoma were observed in fall, suggesting that the larval development would be synchronized with the productivity cycle in the region of Rapa Nui, where maximum chlorophyll concentration is observed during the austral winter. *Corresponding Author E-mail: [email protected] Pacific Science, vol. 71, no. 2 December 9, 2016 (Early view) Introduction The island of Rapa Nui (also known as Easter Island) is part of the Marine and Coastal Protected Area called Coral Nui Nui, Motu Tautara and Hanga Oteo submarine Parks (Sierralta et al. -

ESTADÍSTICO DE PESCA Índice
ANUARIO ESTADÍSTICO DE PESCA Índice INTRODUCCIÓN 7 CAPÍTULO I PRODUCCIÓN PESQUERA 13 CAPÍTULO II INDUSTRIALIZACIÓN 109 CAPÍTULO III COMERCIALIZACIÓN Y CONSUMO 123 CAPÍTULO IV FACTORES DE PRODUCCIÓN 147 CAPÍTULO V NORMATIVIDAD 177 CAPÍTULO VI ESTADÍSTICAS INTERNACIONALES 201 GLOSARIO 237 ÍNDICE DE CUADROS 243 ANEXO 257 SECRETARÍA DE AGRICULTURA, GANADERÍA, DESARROLLO RURAL, PESCA Y ALIMENTACIÓN Javier Bernardo Usabiaga Arroyo SECRETARIO Jerónimo Ramos Sáenz Pardo COMISIONADO NACIONAL DE ACUACULTURA Y PESCA Juan Carlos Cortés García SUBSECRETARIO DE PLANEACIÓN Víctor Villalobos Arámbula SUBSECRETARIO DE AGRICULTURA Y GANADERÍA Antonio Ruiz García SUBSECRETARIO DE DESARROLLO RURAL Mara Angélica Murillo Correa DIRECTORA GENERAL DE POLÍTICA Y FOMENTO PESQUERO Guillermo Compean Jiménez PRESIDENTE DEL INSTITUTO NACIONAL DE LA PESCA p Introducción Introducción a Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación, tiene como uno de sus propósitos esenciales difundir en forma confiable y oportuna, los L principales indicadores de la actividad pesquera en México, que son importantes para conocer el comportamiento y evolución de la explotación, conservación e industrialización de la flora y fauna acuática del país. La SAGARPA a través del desarrollo y actualización de su infraestructura informática y el rediseño de los sistemas estadísticos, aunado a la automatización en sus procesos, propicia las condiciones necesarias para la generación de información estadística actual y confiable, que permite conocer los fenómenos que comprende la pesca en su conjunto. Para la integración de este documento fue necesaria una cercana vinculación entre las delegaciones federales, las oficinas de la SAGARPA y los órganos centrales de la Secretaría, quienes por medio de procedimiento ya establecido, llevaron a cabo la tarea de recopilar e integrar la información estadística emanada de los diferentes agentes que participan activamente en este sector. -

Goldstein Et Al 2019
Journal of Crustacean Biology Advance Access published 24 August 2019 Journal of Crustacean Biology The Crustacean Society Journal of Crustacean Biology 39(5), 574–581, 2019. doi:10.1093/jcbiol/ruz055 Downloaded from https://academic.oup.com/jcb/article-abstract/39/5/574/5554142/ by University of New England Libraries user on 04 October 2019 Development in culture of larval spotted spiny lobster Panulirus guttatus (Latreille, 1804) (Decapoda: Achelata: Palinuridae) Jason S. Goldstein1, Hirokazu Matsuda2, , Thomas R. Matthews3, Fumihiko Abe4, and Takashi Yamakawa4, 1Wells National Estuarine Research Reserve, Maine Coastal Ecology Center, 342 Laudholm Farm Road, Wells, ME 04090 USA; 2Mie Prefecture Fisheries Research Institute, 3564-3, Hamajima, Shima, Mie 517-0404 Japan; 3Florida Fish and Wildlife Conservation Commission, Fish and Wildlife Research Institute, 2796 Overseas Hwy, Suite 119, Marathon, FL 33050 USA; and 4Department of Aquatic Bioscience, Graduate School of Agricultual and Life Sciences, The University of Tokyo, 1-1-1 Yayoi, Bunkyo, Tokyo 113-8657 Japan HeadA=HeadB=HeadA=HeadB/HeadA Correspondence: J.S. Goldstein: e-mail: [email protected] HeadB=HeadC=HeadB=HeadC/HeadB (Received 15 May 2019; accepted 11 July 2019) HeadC=HeadD=HeadC=HeadD/HeadC Ack_Text=DisHead=Ack_Text=HeadA ABSTRACT NList_lc_rparentheses_roman2=Extract1=NList_lc_rparentheses_roman2=Extract1_0 There is little information on the early life history of the spotted spiny lobster Panulirus guttatus (Latreille, 1804), an obligate reef resident, despite its growing importance as a fishery re- BOR_HeadA=BOR_HeadB=BOR_HeadA=BOR_HeadB/HeadA source in the Caribbean and as a significant predator. We cultured newly-hatched P. guttatus BOR_HeadB=BOR_HeadC=BOR_HeadB=BOR_HeadC/HeadB larvae (phyllosomata) in the laboratory for the first time, and the growth, survival, and mor- BOR_HeadC=BOR_HeadD=BOR_HeadC=BOR_HeadD/HeadC phological descriptions are reported through 324 days after hatch (DAH). -

Distribution and Abundance of Panulirus Spp
Bull Mar Sci. 92(2):207–227. 2016 research paper http://dx.doi.org/10.5343/bms.2015.1015 Distribution and abundance of Panulirus spp. phyllosomas off the Mexican Caribbean coast 1 El Colegio de la Frontera Sur Ashanti A Canto-García 1 (ECOSUR–Unidad Chetumal), Jason S Goldstein 2 Departamento de Sistemática 1 * y Ecología Acuática, Av. Eloy Sosa-Cordero Centenario Km 5.5, Chetumal, Laura Carrillo 1 Quintana Roo, Mexico CP 77014. 2 Department of Biological Sciences and School of Marine Sciences and Ocean Engineering, ABSTRACT.—We evaluated the abundance and University of New Hampshire, distribution of Panulirus spp. phyllosomas collected during Durham, New Hampshire 03824. two oceanographic cruises in March 2006 and January * Corresponding author email: 2007 along the Mexican Caribbean coast. In total, 1138 <[email protected]>. phyllosomas were collected in 2006, and 492 were collected in 2007, comprising three families: Palinuridae; Scyllaridae; and Sinaxidae. The most abundant species of Panulirus for both cruises was Panulirus argus (Latreille, 1804). DNA barcoding methods also confirmed the identity of the phyllosomal stages of Panulirus guttatus (Latreille, 1804). The most abundant phyllosomal stages of P. argus in both years were early (I–III) and mid stages (IV–VIII) mostly found near Banco Chinchorro. The least abundant late stages (IX–X) were widely dispersed offshore. During both cruises, >70% of phyllosomal abundance was concentrated in the surface layers (0–50 m). Statistical models indicated that depth, along with year, geographical area, and time of day, were important for early- and mid-stage phyllosoma stages. The distribution and densities of phyllosomas off Quintana Roo differed among years and areas, likely due to varying environmental conditions and mediated by physical processes and the seasonality of reproductive activity of the local and regional spawning stock. -

Taxonomy, Biology and Distribution of Lobsters
Taxonomy, Biology and Distribution of Lobsters 15 Rekha Devi Chakraborty and E.V.Radhakrishnan Crustacean Fisheries Division, Central Marine Fisheries Research Institute, Kochi-682 018 Lobsters are among the most prized of fisheries resources and of significant commercial interest in many countries. Because of their high value and esteemed culinary worth, much attention has been paid to lobsters in biological, fisheries, and systematic literature. They have a great demand in the domestic market as a delicacy and is a foreign exchange earner for the country. Taxonomic status Phylum: Arthropoda Subphylum: Crustacea Class: Malacostraca Subclass: Eumalacostraca Superorder: Eucarida Order: Decapoda Suborder: Macrura Reptantia The suborder Macrura Reptantia consists of three infraorders: Astacidea (Marine lobsters and freshwater crayfishes), Palinuridea (Spiny lobsters and slipper lobsters) and Thalassinidea (mud lobsters). The infraorder Astacidea Summer School on Recent Advances in Marine Biodiversity Conservation and Management 100 Rekha Devi Chakraborty and E.V.Radhakrishnan contains three superfamilies of which only one (the Infraorder Palinuridea, Superfamily Eryonoidea, Family Nephropoidea) is considered here. The remaining two Polychelidae superfamilies (Astacoidea and parastacoidea) contain the 1b. Third pereiopod never with a true chela,in most groups freshwater crayfishes. The superfamily Nephropoidea (40 chelae also absent from first and second pereiopods species) consists almost entirely of commercial or potentially 3a Antennal flagellum reduced to a single broad and flat commercial species. segment, similar to the other antennal segments ..... Infraorder Palinuridea, Superfamily Palinuroidea, The infraorder Palinuridea also contains three superfamilies Family Scyllaridae (Eryonoidea, Glypheoidea and Palinuroidea) all of which are 3b Antennal flagellum long, multi-articulate, flexible, whip- marine. The Eryonoidea are deepwater species of insignificant like, or more rigid commercial interest. -

Guide to the Lobsters and Lobster-Like Animals of Florida, the Gulf Of
Sea Grant Field Guide Series Pl !t p! g jQ4'4JtII Guideto the Lobsters and I obster-like Animals of Florida, the Gulf of Mexico and the CaribbeanRegion Lee Opresko, Dennis Opresko Ronald Thomas and Gilbert Voss Edited and Illustrat.ed by Frederick IH. Bayer University of Ifftami Sea Grant Program hIDAASea Grant No. 04-3-t58-27! Miami, Florl da I973 Foreword The University of Miami Sea Grant Field Guide Series is published to make available to the commercial and sports fishermen, the gen- The University of Miami's Sea Grant Programis a part of the Nationa} eral public, and fisheries and conservation personnel easily usable, SeaGrant Program, which is administered by the National Oceanicand non-technical, well-illustrated guides for the identification of AtmosphericAdministration cf the U.S. Departmentof Conmerce. the marine life of the area. Every means has been used to avoid technical terms where possible. When these must be used to avoid confusion, they are carefully explained and often illustrated. Glossaries are included when thought necessary. But the guides go further than just identification. Where such knowledge is available, information is given on geographical dis- tribution, depth distribution, abundance, time of spawning, present utilization, means of harvesting and mariculture methods, besides other useful information when known. Price: $3.00 The format is uniform in the series for greater ease of use. Actu- al photographs are used where possible but when greater clarity is required, drawings are used. In general we have attempted to illus- trate each species but in cases where two or more species are very similar, this is noted, a single illustration is used, and dis- tinguishing characters are given in the text. -

1 Modeling Spiny Lobster Larval Dispersion in the Tropical Atlantic
Modeling spiny lobster larval dispersion in the tropical Atlantic using satellite data. 1 Camila Aguirre Góes João Antônio Lorenzzetti 1 Douglas F. Marcolino Gherardi 1 Jorge Eduardo Lins Oliveira 2 1 Instituto Nacional de Pesquisas Espaciais - INPE Caixa Postal 515 - 12245-970 - São José dos Campos - SP, Brasil {camila, loren, douglas}@dsr.inpe.br ² Universidade Federal do Rio Grande do Norte - UFRN/DOL Av. Praia de Mãe Luíza s/n; Via Costeira, Cep: 59.014-100 - Natal - RN, Brasil [email protected] Abstract. Spiny lobsters (Palinuridae) have a relatively long planktonic larval phase lasting around one year. Therefore, the mean ocean currents are expected to have a potential for transporting lobster larvae over long distances away from the original spawning areas. We have investigated larval dispersion across tropical Atlantic (20ºN-15ºS; 15ºE-45ºW), using an advection-diffusion model in an attempt to determine the connectivity among different adult stocks. The model updates the position of each larva everyday, along 365 days using the surface geostrophic velocity fields derived from altimeter satellite. Results of simulations indicate that the larval stocks from the African coast do not spread far from the spawning areas. Simulations also indicate that the Brazilian adult stocks could be maintained with larvae released from oceanic islands such as São Pedro and São Paulo Archipelago, Atol das Rocas and Fernando de Noronha. Finally, it is possible that middle South-Atlantic islands (i. e. Ascension Island), could act as stepping-stones between the African and the South-American spiny lobster stocks. Key-words : Larvae dispersion, altimeter satellite, Palinuridae, phyllosoma , advection-diffusion model. -

Marine Ecology Progress Series 469:195
Vol. 469: 195–213, 2012 MARINE ECOLOGY PROGRESS SERIES Published November 26 doi: 10.3354/meps09862 Mar Ecol Prog Ser Contribution to the Theme Section ‘Effects of climate and predation on subarctic crustacean populations’ OPENPEN ACCESSCCESS Ecological role of large benthic decapods in marine ecosystems: a review Stephanie A. Boudreau*, Boris Worm Biology Department, Dalhousie University, 1355 Oxford Street, PO Box 15000, Halifax, Nova Scotia B3H 4R2, Canada ABSTRACT: Large benthic decapods play an increasingly important role in commercial fisheries worldwide, yet their roles in the marine ecosystem are less well understood. A synthesis of exist- ing evidence for 4 infraorders of large benthic marine decapods, Brachyura (true crabs), Anomura (king crabs), Astacidea (clawed lobsters) and Achelata (clawless lobsters), is presented here to gain insight into their ecological roles and possible ecosystem effects of decapod fisheries. The reviewed species are prey items for a wide range of invertebrates and vertebrates. They are omnivorous but prefer molluscs and crustaceans as prey. Experimental studies have shown that decapods influence the structuring of benthic habitat, occasionally playing a keystone role by sup- pressing herbivores or space competitors. Indirectly, via trophic cascades, they can contribute to the maintenance of kelp forest, marsh grass, and algal turf habitats. Changes in the abundance of their predators can strongly affect decapod population trends. Commonly documented non- consumptive interactions include interference-competition for food or shelter, as well as habitat provision for other invertebrates. Anthropogenic factors such as exploitation, the creation of pro- tected areas, and species introductions influence these ecosystem roles by decreasing or increas- ing decapod densities, often with measurable effects on prey communities. -

Two Newly Recorded Species of the Lobster Family Scyllaridae (Thenus Indicus and Scyllarides Haanii) from South of Java, Indonesia
HAYATI Journal of Biosciences 23 (2016) 101e105 HOSTED BY Contents lists available at ScienceDirect HAYATI Journal of Biosciences journal homepage: http://www.journals.elsevier.com/ hayati-journal-of-biosciences Original research article Two Newly Recorded Species of the Lobster Family Scyllaridae (Thenus indicus and Scyllarides haanii) From South of Java, Indonesia * Yusli Wardiatno, Agus Alim Hakim, Ali Mashar, Nurlisa Alias Butet, Luky Adrianto Department of Aquatic Resources Management, Faculty of Fisheries and Marine Science, Bogor Agricultural University, Bogor, West Java, Indonesia. article info abstract Article history: Two species of slipper lobster, Thenus indicus Leach, 1815, and Scyllarides haanii De Haan, 1841, are re- Received 5 February 2016 ported for the first time from the coastal waters of South of Java, part of the Indian Ocean. A total of two Received in revised form specimens, one specimen of T. indicus from Palabuhanratu Bay and one specimen of S. haanii from 30 April 2016 Yogyakarta coastal waters, were collected in April and September 2015, respectively. Descriptions and Accepted 9 May 2016 illustrations of the morphological characteristics of the two species and their habitat are presented. Available online 26 May 2016 Copyright © 2016 Institut Pertanian Bogor. Production and hosting by Elsevier B.V. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/). KEYWORDS: Crustacean, Decapoda, first record, Indian Ocean, slipper lobster 1. Introduction only one species, Thenus orientalis. Taxonomic and biodiversity studies on Indonesian lobsters resulted in the collection of two From a fishery point of view, slipper lobsters of the family different species of family Scyllaridae from South of Java. -

Spiny Lobster (Panulirus Sp.) Life Cycle – a Review and Fisheries Management Implications
Research Notes… Spiny Lobster (Panulirus sp.) Life Cycle – a review and fisheries management implications Léo Barret Abstract The fishery for spiny lobster around the inner granitic islands of the Seychelles has been closed for two consecutive years (2017-2019). Declining trends in indicators of abundance and the loss of coral reef habitats led to the SeyCCAT funded lobster project, aimed at establishing a science-based restoration of commercially important spiny lobster habitats to help develop a sustainable fishery – a project partnership between the University of Seychelles (UniSey), the Seychelles Fishing Authority (SFA), and the Marine Conservation Society Seychelles (MCSS). Spiny lobsters have a complex mero-planktonic lifecycle from a larva living in the open sea to an adult living on the sea floor. The management of the fishery requires a good understanding on all the stages of the spiny lobster life cycle. In this context, the objective of this paper is to provide a short review of the spiny lobster (Panulirus sp.) life cycle and the ecological and fisheries management implications. Introduction (a Seychelles perspective) Spiny lobsters (Palinuridae) are one of the most valuable commercial seafood species globally; for example in Australia, lobster is the country’s most valuable fishery in terms of both overall production and value of export, with a Gross Value of Production of $610 million AUD in 2014 (Plagányi et al., 2018). The FAO annual catch data of spiny (Palinuridae) and clawed (Nephropidae) lobsters has been steadily increasing over the past few decades and the 2017 global catch estimate was 315,469 t (FAO, 2019). Unlike the global trend, Seychelles’ lobster fishery catch has decreased over the past decade, with some of the major challenges being limited recruitment, poaching, and rapid ecosystem change, such as the loss of coral reef habitats due to coral bleaching events.