Downloaded from Genbank for All Australian Palinurid And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

U , '' Regional (;"Ntre of I:::;~, I Central Marine Fisheries Research Institute T - ~ '\~ ~ ~3L'!" ICAR Marine Fisheries P.O
CMFRI S ammelt S ,4(J(Jt (JU Recent Advances in Se"ed Production and Growout Techniques for Marine Finfish and Shellfish ,1 I Compiled and Edited by Dr. G.Gopakumar, Principal Scientist & Director, Summer School and '. Dr. Boby Ignatius, Scientist (SS) Central Marine Fisheries Research Institute j' U , '' Regional (;"ntre of i:::;~, I Central Marine Fisheries Research Institute t - ~ '\~ ~ ~3l'!" ICAR Marine Fisheries P.O . Tamil Nadu - 623 520 ' ~~ ... ~"'~~ SEED PRODUCTION OF THE SAND LOBSTER THENUS ORIENTALIS (LUND) Joe K. Kizhakudan Research Centre of CMFRI, Chennai 3f With the decline in many commercial fisheries worldwide and an ever increasing demand for seafood protein, there is a growing need for augmenting the production of high-protein, high-value resources like lobsters. Aquaculture remains the ideal measure to augment production and ensure conseNation, and even enhancement. of natural stocks. Aquaculture provides a two-pronged solution towards increasing the fish production through ., farming of hatchery-produced seed of commercially important finfishes and shellfishes , enhancing natural stocks by sea ranching hatchery-produced seed of commercially important finfishes and shellfishes Lobsters are among the most priced seafood delicacies enjoying a special demand in international markets. As against a world average annual productio'n of2.1 lakh tonnes, India's average annual lobster production is about 2000 tonnes. With the distinction of being perhaps, the only seafood resource in India's trade economy, which remains relatively low down the ladder in terms of quantity of production but brings in maximum foreign exchange, lobsters have been the subject of study for more than two decades now. -

Lobsters-Identification, World Distribution, and U.S. Trade
Lobsters-Identification, World Distribution, and U.S. Trade AUSTIN B. WILLIAMS Introduction tons to pounds to conform with US. tinents and islands, shoal platforms, and fishery statistics). This total includes certain seamounts (Fig. 1 and 2). More Lobsters are valued throughout the clawed lobsters, spiny and flat lobsters, over, the world distribution of these world as prime seafood items wherever and squat lobsters or langostinos (Tables animals can also be divided rougWy into they are caught, sold, or consumed. 1 and 2). temperate, subtropical, and tropical Basically, three kinds are marketed for Fisheries for these animals are de temperature zones. From such partition food, the clawed lobsters (superfamily cidedly concentrated in certain areas of ing, the following facts regarding lob Nephropoidea), the squat lobsters the world because of species distribu ster fisheries emerge. (family Galatheidae), and the spiny or tion, and this can be recognized by Clawed lobster fisheries (superfamily nonclawed lobsters (superfamily noting regional and species catches. The Nephropoidea) are concentrated in the Palinuroidea) . Food and Agriculture Organization of temperate North Atlantic region, al The US. market in clawed lobsters is the United Nations (FAO) has divided though there is minor fishing for them dominated by whole living American the world into 27 major fishing areas for in cooler waters at the edge of the con lobsters, Homarus americanus, caught the purpose of reporting fishery statis tinental platform in the Gul f of Mexico, off the northeastern United States and tics. Nineteen of these are marine fish Caribbean Sea (Roe, 1966), western southeastern Canada, but certain ing areas, but lobster distribution is South Atlantic along the coast of Brazil, smaller species of clawed lobsters from restricted to only 14 of them, i.e. -

B Chromosomes, Ribosomal Genes and Telomeric Sequences
Genetica DOI 10.1007/s10709-012-9691-4 Comparative cytogenetics in four species of Palinuridae: B chromosomes, ribosomal genes and telomeric sequences Susanna Salvadori • Elisabetta Coluccia • Federica Deidda • Angelo Cau • Rita Cannas • Anna Maria Deiana Received: 18 May 2012 / Accepted: 20 November 2012 Ó Springer Science+Business Media Dordrecht 2012 Abstract The evolutionary pathway of Palinuridae Introduction (Crustacea, Decapoda) is still controversial, uncertain and unexplored, expecially from a karyological point of view. Scyllaridae and Palinuridae constitute the Achelata group, Here we describe the South African spiny lobster Jasus considered to be monophyletic and the Palinuridae now lalandii karyotype: n and 2n values, heterochromatin dis- includes the Sinaxidae family (Patek et al. 2006; George tribution, nucleolar organizer region (NOR) location and 2006; Groeneweld et al. 2007; Palero et al. 2009a; Tsang telomeric repeat structure and location. To compare the et al. 2009). genomic and chromosomal organization in Palinuridae we Fossil records from North America, Europe and Aus- located NORs in Panulirus regius, Palinurus gilchristi and tralia suggest that the Palinuridae arose in the early Palinurus mauritanicus: all species showed multiple Mesozoic (George 2006). The family currently comprises NORs. In J. lalandii NORs were located on three chro- approximately ten genera and fifty species (including those mosome pairs, with interindividual polymorphism. In of the Synaxidae), which can be subdivided into two P. regius andinthetwoPalinurus species NORs were located groups: the Stridentes, and the Silentes. This family has on two chromosome pairs. In the two last species 45S received much attention and there are data on comparative ribosomal gene loci were also found on B chromosomes. -
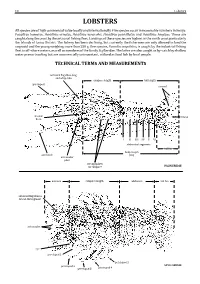
Lobsters LOBSTERS§
18 Lobsters LOBSTERS§ All species are of high commercial value locally and internationally. Five species occur in reasonable numbers in Kenya: Panulirus homarus, Panulirus ornatus, Panulirus versicolor, Panulirus penicillatus and Panulirus longipes. These are caughtungravid along and the the coast young by weighing the artisanal more fishing than 250 fleet. g. Landings One species, of these Puerulus species angulatus are highest in the north coast particularly the Islands of Lamu District. The fishery has been declining,Scyllaridae. but currently The latter the fishermen are also caught are only as by–catch allowed toby landshallow the , is caught by the industrial fishing fleet in off–shore waters, as well as members of the family water prawn trawling but areTECHNICAL commercially unimportant, TERMS AND utilized MEASUREMENTS as food fish by local people. and whip–like antennal flagellum long carapace length tail length pereiopod uropod frontal telson horn III III IV VIV abdominal segments tail fan body length antennule (BL) antennular plate strong spines on carapace PALINURIDAE antenna carapace length abdomen tail fan antennal flagellum a broad, flat segment antennules eye pereiopod 1 pereiopod 5 pereiopod 2 SCYLLARIDAE pereiopod 3 pereiopod 4 Guide to Families 19 GUIDE TO FAMILIES NEPHROPIDAE Page 20 True lobsters § To about 15 cm. Marine, mainly deep waters on soft included in the Guide to Species. 1st pair of substrates. Three species of interest to fisheriespereiopods are large 3rd pair of pereiopods with chela PALINURIDAE Page 21 Antennal Spiny lobsters § To about 50 cm. Marine, mostly shallow waters on flagellum coral and sand stone reefs, some species on soft included in the Guide to Species. -

Title NOTES on the NAUPLIOSOMA and NEWLY HATCHED
NOTES ON THE NAUPLIOSOMA AND NEWLY Title HATCHED PHYLLOSOMA OF IBACUS CILIATUS (VON SIEBOLD) Author(s) Harada, Eiji PUBLICATIONS OF THE SETO MARINE BIOLOGICAL Citation LABORATORY (1958), 7(1): 173-180 Issue Date 1958-12-20 URL http://hdl.handle.net/2433/174595 Right Type Departmental Bulletin Paper Textversion publisher Kyoto University NOTES ON THE NAUPLIOSOMA AND NEWLY HATCHED PHYLLOSOMA OF IBACUS CILIATUS 1 (VON SIEBOLD) ) EIJI HARADA Seto Marine Biological Laboratory, Sirahama With Plates IX-XI and 2 Text-figures Introduction The purpose of the present paper is to afford some contributions to the post-larval development of a common scyllarid lobster Ibacus ciliatus (voN SIEBOLD), together with some knowledge on the biology of its larvae. For our knowledge of the phyllosoma larvae of Ibacus ciliatus, we have the reports of many investigators, such as DE HAAN (1833-1850), RIGHTERS (1873), BALSS (1914), STEPHENSEN (1923), GURNEY (1936), TOKIOKA (1954), etc., but if these re ports are consulted, it may clearly be shown that the phyllosomas which were treated and described in these reports are all of the later developmental stages, and no case in the earlier stages of the phyllosoma is available. This may be mainly due to that the phyllosomas in these reports are those largely obtained in the field by chance or during the courses of large-scaled oceanographical investigations. This may also be met with in the case if we take the phyllosomas of other species into account. For instance, if one intends to make comparison of the newly hatched phyllosomas of certain species, one will be sure to find a number of species of which the newly hatched phyllosoma is undescribed. -

The Utilization of Lobsters by Humans in the Mediterranean Basin from the Prehistoric Era to the Modern Era – an Interdisciplinary Short Review
Athens Journal of Mediterranean Studies- Volume 1, Issue 3 – Pages 223-234 The Utilization of Lobsters by Humans in the Mediterranean Basin from the Prehistoric Era to the Modern Era – An Interdisciplinary Short Review By Ehud Spanier The Mediterranean and Red Seas host a variety of clawed, spiny and slipper lobsters. Lobsters' utilization in ancient times varied, ranging from complete prohibition by the Jewish religion, to that of epicurean status in the Roman world. One of the earliest known illustrations of a spiny lobster was a wall carving in Egypt depicting the Queen Hatshepsut expedition to the Red Sea in the 15th century BC. Lobsters were known by the ancient Greeks and Romans as was expressed in art forms and writings. Lobsters also appeared in ancient mosaics and coins. The writings of naturalists and philosophers from the Roman-Hellenistic period, together with illustrative records indicate that lobsters were a popular food and there was considerable knowledge of their classification, biology and fisheries. The popularity of lobsters as gourmet food increased with time followed by an expansion of the scientific knowledge as well as over exploitation of these resources. Keywords: Antiquity, Biology, Fisheries, Lobsters, Mediterranean. Introduction A variety of edible lobsters, that are still commercially significant to human inhabitants today, are found in the water of the Mediterranean and adjacent Red Sea regions. This important marine resource includes at least two Mediterranean clawed lobsters (the European lobster, Homarus gammarus, and the Norway lobster, Nephrops norvegicus), five spiny lobsters (two in the Mediterranean: the common spiny lobster, Palinurus elephas, and the pink spiny lobster, P. -

On the Structure and Mechanism of the Gastric Mill in Decapoda
ON THE STRUCTURE AND MECHANISM OF THE GASTRIC MILL IN DECAPODA. IV. The Structure of the Gastric Mill in Reptantous Macrura. By S. S. PATWARDHAN, M.Sc., Department of Zoology, College of Science, Nagpur. Received January 25, 1935. (Communicated by Prof. M. A. Moghe, M.A., M.Sc., r.z.s.) 1. Introduction. THE sub-order Macrura may be divided into two groups: (1) Reptantous Macrura, comprising crayfishes, lobsters and allied forms and characterised by possessing a dorsoventrally flattened body and a crawling and climbing mode of locomotion, and (2) Natantous Macrura comprising prawns and shrimps, characterised by possessing a laterally flattened body and a swimming mode of locomotion. The Reptantous Macrura are also characterised by the universal presence of a complex gastric mill and simple mandibles. In the present communication it is proposed to give a comparative account of the gastric mill in Reptantous Macrura. 2. Material and Method. The gastric mills of crayfish, Astacus fluviatilis, Palinurus and of many other lobsters have been already described in many text-books (Huxley, 1880; Powell, 1913) . The material at my disposal consists of six species obtained from various Biological Supplies Stations and represents all the tribes, excepting Eryonidea, and four families. Tribe Family Nephropsidea N ephropsidae Homarus vulgaris* M. Edw. Nephrops norvegicusf Leach. Loricata Palinuridee Palinurus vulgaris* Latr. Scyllaridee Scyllarus arctus Pabr. Thalassinidea Callianassidae Callianassa subterraneat Leach. Gebia littoralist Desm. Two or three specimens of each type were examined and were treated with Caustic Potash as I did in Anomura. A brief account of the cardiac * From Plymouth. t From Naples. -

First Record and Description of Panulirus Pascuensis (Reed, 1954) First Stage Phyllosoma in the Plankton of Rapa Nui
First record and description of Panulirus pascuensis (Reed, 1954) first stage phyllosoma in the plankton of Rapa Nui By Meerhoff Erika*, Mujica Armando, García Michel, and Nava María Luisa Abstract Among the most striking crustaceans from Rapa Nui Island (27° S, 109°22’ W) is the endemic lobster Panulirus pascuensis, commonly known as the spiny lobster. This species is also present in Pitcairn Island and the Salas y Gomez ridge. The larvae of this species pass through different phyllosoma stages until metamorphosis, in which they molt to puerulus, a transitional stage adapted to benthic life. This species is an important fishery resource for the inhabitants of Rapa Nui. However, there are several gaps in the biological knowledge of this species, including its ontogeny. We sampled zooplankton to obtain first stage P. pascuensis phyllosoma during three oceanographic campaigns around Rapa Nui (April 2015, September 2015 and March 2016), individuals were encountered between the surface and 200 m depth with an abundance of 1.3 indiv/1000 m3. These individuals, along with laboratory hatched phyllosoma, allowed us to describe the morphology of this larval stage. The P. pascuensis stage I phyllosoma were observed in fall, suggesting that the larval development would be synchronized with the productivity cycle in the region of Rapa Nui, where maximum chlorophyll concentration is observed during the austral winter. *Corresponding Author E-mail: [email protected] Pacific Science, vol. 71, no. 2 December 9, 2016 (Early view) Introduction The island of Rapa Nui (also known as Easter Island) is part of the Marine and Coastal Protected Area called Coral Nui Nui, Motu Tautara and Hanga Oteo submarine Parks (Sierralta et al. -

American Samoa Archipelago Fishery Ecosystem Plan 2017
ANNUAL STOCK ASSESSMENT AND FISHERY EVALUATION REPORT: AMERICAN SAMOA ARCHIPELAGO FISHERY ECOSYSTEM PLAN 2017 Western Pacific Regional Fishery Management Council 1164 Bishop St., Suite 1400 Honolulu, HI 96813 PHONE: (808) 522-8220 FAX: (808) 522-8226 www.wpcouncil.org The ANNUAL STOCK ASSESSMENT AND FISHERY EVALUATION REPORT for the AMERICAN SAMOA ARCHIPELAGO FISHERY ECOSYSTEM PLAN 2017 was drafted by the Fishery Ecosystem Plan Team. This is a collaborative effort primarily between the Western Pacific Regional Fishery Management Council, NMFS-Pacific Island Fisheries Science Center, Pacific Islands Regional Office, Division of Aquatic Resources (HI) Department of Marine and Wildlife Resources (AS), Division of Aquatic and Wildlife Resources (Guam), and Division of Fish and Wildlife (CNMI). This report attempts to summarize annual fishery performance looking at trends in catch, effort and catch rates as well as provide a source document describing various projects and activities being undertaken on a local and federal level. The report also describes several ecosystem considerations including fish biomass estimates, biological indicators, protected species, habitat, climate change, and human dimensions. Information like marine spatial planning and best scientific information available for each fishery are described. This report provides a summary of annual catches relative to the Annual Catch Limits established by the Council in collaboration with the local fishery management agencies. Edited By: Marlowe Sabater, Asuka Ishizaki, Thomas Remington, and Sylvia Spalding, WPRFMC. This document can be cited as follows: WPRFMC, 2018. Annual Stock Assessment and Fishery Evaluation Report for the American Samoa Archipelago Fishery Ecosystem Plan 2017. Sabater, M., Ishizaki, A., Remington, T., Spalding, S. (Eds.) Western Pacific Regional Fishery Management Council. -

ESTADÍSTICO DE PESCA Índice
ANUARIO ESTADÍSTICO DE PESCA Índice INTRODUCCIÓN 7 CAPÍTULO I PRODUCCIÓN PESQUERA 13 CAPÍTULO II INDUSTRIALIZACIÓN 109 CAPÍTULO III COMERCIALIZACIÓN Y CONSUMO 123 CAPÍTULO IV FACTORES DE PRODUCCIÓN 147 CAPÍTULO V NORMATIVIDAD 177 CAPÍTULO VI ESTADÍSTICAS INTERNACIONALES 201 GLOSARIO 237 ÍNDICE DE CUADROS 243 ANEXO 257 SECRETARÍA DE AGRICULTURA, GANADERÍA, DESARROLLO RURAL, PESCA Y ALIMENTACIÓN Javier Bernardo Usabiaga Arroyo SECRETARIO Jerónimo Ramos Sáenz Pardo COMISIONADO NACIONAL DE ACUACULTURA Y PESCA Juan Carlos Cortés García SUBSECRETARIO DE PLANEACIÓN Víctor Villalobos Arámbula SUBSECRETARIO DE AGRICULTURA Y GANADERÍA Antonio Ruiz García SUBSECRETARIO DE DESARROLLO RURAL Mara Angélica Murillo Correa DIRECTORA GENERAL DE POLÍTICA Y FOMENTO PESQUERO Guillermo Compean Jiménez PRESIDENTE DEL INSTITUTO NACIONAL DE LA PESCA p Introducción Introducción a Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación, tiene como uno de sus propósitos esenciales difundir en forma confiable y oportuna, los L principales indicadores de la actividad pesquera en México, que son importantes para conocer el comportamiento y evolución de la explotación, conservación e industrialización de la flora y fauna acuática del país. La SAGARPA a través del desarrollo y actualización de su infraestructura informática y el rediseño de los sistemas estadísticos, aunado a la automatización en sus procesos, propicia las condiciones necesarias para la generación de información estadística actual y confiable, que permite conocer los fenómenos que comprende la pesca en su conjunto. Para la integración de este documento fue necesaria una cercana vinculación entre las delegaciones federales, las oficinas de la SAGARPA y los órganos centrales de la Secretaría, quienes por medio de procedimiento ya establecido, llevaron a cabo la tarea de recopilar e integrar la información estadística emanada de los diferentes agentes que participan activamente en este sector. -

Goldstein Et Al 2019
Journal of Crustacean Biology Advance Access published 24 August 2019 Journal of Crustacean Biology The Crustacean Society Journal of Crustacean Biology 39(5), 574–581, 2019. doi:10.1093/jcbiol/ruz055 Downloaded from https://academic.oup.com/jcb/article-abstract/39/5/574/5554142/ by University of New England Libraries user on 04 October 2019 Development in culture of larval spotted spiny lobster Panulirus guttatus (Latreille, 1804) (Decapoda: Achelata: Palinuridae) Jason S. Goldstein1, Hirokazu Matsuda2, , Thomas R. Matthews3, Fumihiko Abe4, and Takashi Yamakawa4, 1Wells National Estuarine Research Reserve, Maine Coastal Ecology Center, 342 Laudholm Farm Road, Wells, ME 04090 USA; 2Mie Prefecture Fisheries Research Institute, 3564-3, Hamajima, Shima, Mie 517-0404 Japan; 3Florida Fish and Wildlife Conservation Commission, Fish and Wildlife Research Institute, 2796 Overseas Hwy, Suite 119, Marathon, FL 33050 USA; and 4Department of Aquatic Bioscience, Graduate School of Agricultual and Life Sciences, The University of Tokyo, 1-1-1 Yayoi, Bunkyo, Tokyo 113-8657 Japan HeadA=HeadB=HeadA=HeadB/HeadA Correspondence: J.S. Goldstein: e-mail: [email protected] HeadB=HeadC=HeadB=HeadC/HeadB (Received 15 May 2019; accepted 11 July 2019) HeadC=HeadD=HeadC=HeadD/HeadC Ack_Text=DisHead=Ack_Text=HeadA ABSTRACT NList_lc_rparentheses_roman2=Extract1=NList_lc_rparentheses_roman2=Extract1_0 There is little information on the early life history of the spotted spiny lobster Panulirus guttatus (Latreille, 1804), an obligate reef resident, despite its growing importance as a fishery re- BOR_HeadA=BOR_HeadB=BOR_HeadA=BOR_HeadB/HeadA source in the Caribbean and as a significant predator. We cultured newly-hatched P. guttatus BOR_HeadB=BOR_HeadC=BOR_HeadB=BOR_HeadC/HeadB larvae (phyllosomata) in the laboratory for the first time, and the growth, survival, and mor- BOR_HeadC=BOR_HeadD=BOR_HeadC=BOR_HeadD/HeadC phological descriptions are reported through 324 days after hatch (DAH). -

Distribution and Abundance of Panulirus Spp
Bull Mar Sci. 92(2):207–227. 2016 research paper http://dx.doi.org/10.5343/bms.2015.1015 Distribution and abundance of Panulirus spp. phyllosomas off the Mexican Caribbean coast 1 El Colegio de la Frontera Sur Ashanti A Canto-García 1 (ECOSUR–Unidad Chetumal), Jason S Goldstein 2 Departamento de Sistemática 1 * y Ecología Acuática, Av. Eloy Sosa-Cordero Centenario Km 5.5, Chetumal, Laura Carrillo 1 Quintana Roo, Mexico CP 77014. 2 Department of Biological Sciences and School of Marine Sciences and Ocean Engineering, ABSTRACT.—We evaluated the abundance and University of New Hampshire, distribution of Panulirus spp. phyllosomas collected during Durham, New Hampshire 03824. two oceanographic cruises in March 2006 and January * Corresponding author email: 2007 along the Mexican Caribbean coast. In total, 1138 <[email protected]>. phyllosomas were collected in 2006, and 492 were collected in 2007, comprising three families: Palinuridae; Scyllaridae; and Sinaxidae. The most abundant species of Panulirus for both cruises was Panulirus argus (Latreille, 1804). DNA barcoding methods also confirmed the identity of the phyllosomal stages of Panulirus guttatus (Latreille, 1804). The most abundant phyllosomal stages of P. argus in both years were early (I–III) and mid stages (IV–VIII) mostly found near Banco Chinchorro. The least abundant late stages (IX–X) were widely dispersed offshore. During both cruises, >70% of phyllosomal abundance was concentrated in the surface layers (0–50 m). Statistical models indicated that depth, along with year, geographical area, and time of day, were important for early- and mid-stage phyllosoma stages. The distribution and densities of phyllosomas off Quintana Roo differed among years and areas, likely due to varying environmental conditions and mediated by physical processes and the seasonality of reproductive activity of the local and regional spawning stock.