Decapod and Stomatopod Crustacea from Ascension Island, South Atlantic Ocean
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Taxonomic Study of the Pagurus Forbesii "Complex" (Crustacea
Taxonomic study of the Pagurus forbesii "complex" (Crustacea: Decapoda: Paguridae). Description of Pagurus pseudosculptimanus sp. nov. from Alborán Sea (Southern Spain, Western Mediterranean Sea). GARCÍA MUÑOZ J.E.1, CUESTA J.A.2 & GARCÍA RASO J.E.1* 1 Dept. Biología Animal, Fac. Ciencias, Univ. Málaga, Campus de Teatinos s/n, 29071 Málaga, Spain. 2 Inst. Ciencias Marinas de Andalucía (CSIC), Av. República Saharaui, 2, 11519 Puerto Real, Cádiz, Spain. * Corresponding author - e-mail address: [email protected] ABSTRACT The study of hermit crabs from Alboran Sea has allowed recognition of two different morphological forms under what had been understood as Pagurus forbesii. Based on morphological observations with various species of Pagurus, and molecular studies, a new species is defined and described as P. pseudosculptimanus. An overview on species of Pagurus from the eastern Atlantic and Mediterranean Sea is provided. Key words: Pagurus, new species, Mediterranean, eastern Atlantic. 1 Introduction More than 170 species from around the world are currently assigned to the genus Pagurus Fabricius, 1775 (Lemaitre and Cruz Castaño 2004; Mantelatto et al. 2009; McLaughlin 2003, McLaughlin et al. 2010). This genus is complex because of there is high morphological variability and similarity among some species, and has been divided in groups (e.g. Lemaitre and Cruz Castaño 2004 for eastern Pacific species; Ingle, 1985, for European species) with difficulty (Ayón-Parente and Hendrickx 2012). This difficulty has lead to taxonomic problems, although molecular techniques have been recently used to elucidate some species (Mantelatto et al. 2009; Da Silva et al. 2011). Thirteen species are present in eastern Atlantic (European and the adjacent African waters) (Ingle 1993; Udekem d'Acoz 1999; Froglia, 2010, MarBEL Data System - Türkay 2012, García Raso et al., in press) but only nine of these (the first ones mentioned below) have been cited in the Mediterranean Sea, all of them are present in the study area (Alboran Sea, southern Spain). -

East and Central Africa 19
Most countries have based their long-term planning (‘vision’) documents on harnessing science, technology and innovation to development. Kevin Urama, Mammo Muchie and Remy Twingiyimana A schoolboy studies at home using a book illuminated by a single electric LED lightbulb in July 2015. Customers pay for the solar panel that powers their LED lighting through regular instalments to M-Kopa, a Nairobi-based provider of solar-lighting systems. Payment is made using a mobile-phone money-transfer service. Photo: © Waldo Swiegers/Bloomberg via Getty Images 498 East and Central Africa 19 . East and Central Africa Burundi, Cameroon, Central African Republic, Chad, Comoros, Congo (Republic of), Djibouti, Equatorial Guinea, Eritrea, Ethiopia, Gabon, Kenya, Rwanda, Somalia, South Sudan, Uganda Kevin Urama, Mammo Muchie and Remy Twiringiyimana Chapter 19 INTRODUCTION which invest in these technologies to take a growing share of the global oil market. This highlights the need for oil-producing Mixed economic fortunes African countries to invest in science and technology (S&T) to Most of the 16 East and Central African countries covered maintain their own competitiveness in the global market. in the present chapter are classified by the World Bank as being low-income economies. The exceptions are Half the region is ‘fragile and conflict-affected’ Cameroon, the Republic of Congo, Djibouti and the newest Other development challenges for the region include civil strife, member, South Sudan, which joined its three neighbours religious militancy and the persistence of killer diseases such in the lower middle-income category after being promoted as malaria and HIV, which sorely tax national health systems from low-income status in 2014. -
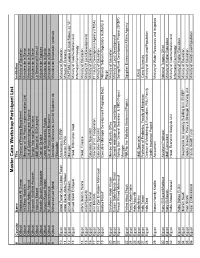
Cairo Workshop Participants
Master Cairo Workshop Participant List # Country Name Title Institution 1 Djibouti Abdourazak Ali Osman Director of Planning Department Ministry of Education 2 Djibouti Ali Sillaye Abdallah Manager of the Project Implementation Unit Ministere de la Sante 3 Djibouti Ammar Abdou Ahmed Dri. of Epidemiology and Hygiene Ministere de la Sante 4 Djibouti Assoweh Abdillahi Assoweh Service Information Sanitaire Ministere de la Sante 5 Djibouti Fatouma Bakard M&E Specialist, SIDA project Le Secretariat Executif 6 Djibouti Housein Doualeh Aboubaker Chef de Service Ministere de Finances 7 Djibouti Hussein Kayad Halane Unite de Gestion de Projets Ministere de la Sante 8 Djibouti M. Abdelrahmane Dir. of Planning and Research Ministere de la Sante 9 Djibouti Mohamed Issé Mahdi Secrétaire Générale du Comité Supérieur de Ministère de l'éducation nationale l'Education 10 Egypt Abdel Fattah Samir Abdel Fattah Accountant, ECEEP Ministry of Education 11 Egypt Abdel Samie Abdel Hafeez Director PPMU Ministry of Economy 12 Egypt Ahmed Abdel Monem Manager PAPFAM, League of Arab States, 22 "A" 13 Egypt Ahmed Saad El Sayed Head, Information Dept. Ministry of Communication and InformationTechnology: 14 Egypt Alfons Ibrahim Hanna Head, Finance Ministry of Education 15 Egypt Amal Sayed Ali Ministry Of Local Development 16 Egypt Amany Kamel Education Specialist Ministry of Education 17 Egypt Amr Mostafa Director of Int. Cooperation IT Industry Development Agency (ITIDA) 18 Egypt Amr Zein El-Abdein Mahmoud Education Specialist Ministry of Education 19 Egypt Bodour Nassif Executive Manager Development Programs Dept. National Telecom Regulatory Authority in Egypt 20 Egypt Ebraheem Abdel Khalek Member of Quality Office Ministry of Education 21 Egypt Essam Galal Hassan Shaat General manager of local monitoring Ministry of Local Development 22 Egypt Farouk Ahmed Mahmoud Sohag Gov. -

Decapod Crustacean Grooming: Functional Morphology, Adaptive Value, and Phylogenetic Significance
Decapod crustacean grooming: Functional morphology, adaptive value, and phylogenetic significance N RAYMOND T.BAUER Center for Crustacean Research, University of Southwestern Louisiana, USA ABSTRACT Grooming behavior is well developed in many decapod crustaceans. Antennular grooming by the third maxillipedes is found throughout the Decapoda. Gill cleaning mechanisms are qaite variable: chelipede brushes, setiferous epipods, epipod-setobranch systems. However, microstructure of gill cleaning setae, which are equipped with digitate scale setules, is quite conservative. General body grooming, performed by serrate setal brushes on chelipedes and/or posterior pereiopods, is best developed in decapods at a natant grade of body morphology. Brachyuran crabs exhibit less body grooming and virtually no specialized body grooming structures. It is hypothesized that the fouling pressures for body grooming are more severe in natant than in replant decapods. Epizoic fouling, particularly microbial fouling, and sediment fouling have been shown r I m ans of amputation experiments to produce severe effects on olfactory hairs, gills, and i.icubated embryos within short lime periods. Grooming has been strongly suggested as an important factor in the coevolution of a rhizocephalan parasite and its anomuran host. The behavioral organization of grooming is poorly studied; the nature of stimuli promoting grooming is not understood. Grooming characters may contribute to an understanding of certain aspects of decapod phylogeny. The occurrence of specialized antennal grooming brushes in the Stenopodidea, Caridea, and Dendrobranchiata is probably not due to convergence; alternative hypotheses are proposed to explain the distribution of this grooming character. Gill cleaning and general body grooming characters support a thalassinidean origin of the Anomura; the hypothesis of brachyuran monophyly is supported by the conservative and unique gill-cleaning method of the group. -

Marine ==- Biology © Springer-Verlag 1988
Marine Biology 98, 39-49 (1988) Marine ==- Biology © Springer-Verlag 1988 Analysis of the structure of decapod crustacean assemblages off the Catalan coast (North-West Mediterranean) P. Abell6, F.J. Valladares and A. Castell6n Institute de Ciencias del Mar, Passeig Nacional s/n, E-08003 Barcelona, Spain Abstract Zariquiey Alvarez 1968, Garcia Raso 1981, 1982, 1984), as well as different biological aspects of the economically We sampled the communities of decapod crustaceans important species (Sarda 1980, Sarda etal. 1981, etc.). inhabiting the depth zone between 3 and 871 m off the More recently, some studies of the species distribution of Catalan coast (North-West Mediterranean) from June the decapod crustacean communities of the North-West 1981 to June 1983. The 185 samples comprised 90 species Mediterranean have been published (Sarda and Palo- differing widely in their depth distributions. Multivariate mera 1981, Castellon and Abello 1983, Carbonell 1984, analysis revealed four distinct faunistic assemblages, (1) Abello 1986). However, the quantitative composition of littoral communities over sandy bottoms, (2) shelf com the decapod crustacean communities of this area remain munities over terrigenous muds, (3) upper-slope com largely unknown, and comparable efforts to those of munities, and (4) lower-slope or bathyal communities. The Arena and Li Greci (1973), Relini (1981), or Tunesi (1986) brachyuran crab Liocarcinus depurator is the most abun are lacking. dant species of the shelf assemblage, although L. vernalis The present -

Caribbean Wildlife Undersea 2017
Caribbean Wildlife Undersea life This document is a compilation of wildlife pictures from The Caribbean, taken from holidays and cruise visits. Species identification can be frustratingly difficult and our conclusions must be checked via whatever other resources are available. We hope this publication may help others having similar problems. While every effort has been taken to ensure the accuracy of the information in this document, the authors cannot be held re- sponsible for any errors. Copyright © John and Diana Manning, 2017 1 Angelfishes (Pomacanthidae) Corals (Cnidaria, Anthozoa) French angelfish 7 Bipinnate sea plume 19 (Pomacanthus pardu) (Antillogorgia bipinnata) Grey angelfish 8 Black sea rod 20 (Pomacanthus arcuatus) (Plexaura homomalla) Queen angelfish 8 Blade fire coral 20 (Holacanthus ciliaris) (Millepora complanata) Rock beauty 9 Branching fire coral 21 (Holacanthus tricolor) (Millepora alcicornis) Townsend angelfish 9 Bristle Coral 21 (Hybrid) (Galaxea fascicularis) Elkhorn coral 22 Barracudas (Sphyraenidae) (Acropora palmata) Great barracuda 10 Finger coral 22 (Sphyraena barracuda) (Porites porites) Fire coral 23 Basslets (Grammatidae) (Millepora dichotoma) Fairy basslet 10 Great star coral 23 (Gramma loreto) (Montastraea cavernosa) Grooved brain coral 24 Bonnetmouths (Inermiidae) (Diploria labyrinthiformis) Boga( Inermia Vittata) 11 Massive starlet coral 24 (Siderastrea siderea) Bigeyes (Priacanthidae) Pillar coral 25 Glasseye snapper 11 (Dendrogyra cylindrus) (Heteropriacanthus cruentatus) Porous sea rod 25 (Pseudoplexaura -

Checklists of Crustacea Decapoda from the Canary and Cape Verde Islands, with an Assessment of Macaronesian and Cape Verde Biogeographic Marine Ecoregions
Zootaxa 4413 (3): 401–448 ISSN 1175-5326 (print edition) http://www.mapress.com/j/zt/ Article ZOOTAXA Copyright © 2018 Magnolia Press ISSN 1175-5334 (online edition) https://doi.org/10.11646/zootaxa.4413.3.1 http://zoobank.org/urn:lsid:zoobank.org:pub:2DF9255A-7C42-42DA-9F48-2BAA6DCEED7E Checklists of Crustacea Decapoda from the Canary and Cape Verde Islands, with an assessment of Macaronesian and Cape Verde biogeographic marine ecoregions JOSÉ A. GONZÁLEZ University of Las Palmas de Gran Canaria, i-UNAT, Campus de Tafira, 35017 Las Palmas de Gran Canaria, Spain. E-mail: [email protected]. ORCID iD: 0000-0001-8584-6731. Abstract The complete list of Canarian marine decapods (last update by González & Quiles 2003, popular book) currently com- prises 374 species/subspecies, grouped in 198 genera and 82 families; whereas the Cape Verdean marine decapods (now fully listed for the first time) are represented by 343 species/subspecies with 201 genera and 80 families. Due to changing environmental conditions, in the last decades many subtropical/tropical taxa have reached the coasts of the Canary Islands. Comparing the carcinofaunal composition and their biogeographic components between the Canary and Cape Verde ar- chipelagos would aid in: validating the appropriateness in separating both archipelagos into different ecoregions (Spalding et al. 2007), and understanding faunal movements between areas of benthic habitat. The consistency of both ecoregions is here compared and validated by assembling their decapod crustacean checklists, analysing their taxa composition, gath- ering their bathymetric data, and comparing their biogeographic patterns. Four main evidences (i.e. different taxa; diver- gent taxa composition; different composition of biogeographic patterns; different endemicity rates) support that separation, especially in coastal benthic decapods; and these parametres combined would be used as a valuable tool at comparing biotas from oceanic archipelagos. -

2010-11-23-MO-DEA-Kalaupapa
National Park Service U.S. Department of the Interior Kalaupapa National Historical Park Hawaii Project to Repair the Kalaupapa Dock Structures Environmental Assessment August 2010 EXECUTIVE SUMMARY The Kalaupapa Settlement is home to surviving Hansen's disease (leprosy) patients, and is cur- rently managed jointly by the Hawaii Department of Health and the National Park Service (NPS). The vast majority of materials needed to sustain the park and the Kalaupapa Settlement is received by barge delivery. An engineering study (Daly 2005) has determined that severe win- ter swell conditions have compromised the structural integrity of the Kalaupapa harbor facilities used by the barge. The NPS proposes to ensure delivery of supplies essential to operate and maintain Kalaupapa National Historical Park (“the park”) and the community by improving conditions of the dock structures at the harbor. This environmental assessment considered two alternatives for improving conditions of the dock structures: Alternative A: The No Action Alternative: Current NPS management operations at the dock and harbor would remain unchanged without repair to the dock structures. The integrity and stability of the pier may be compromised to the point of being unsafe for barge operations. Over the long-term, barge service to the park would likely be disrupted or become sporadic. Delive- ries of annual supplies and materials used for state operations, park programs, and the park’s ongoing rehabilitation of historic properties would be affected. Alternative B: The Preferred Alternative: This alternative would include completion of the repairs necessary to maintain service via a small barge. Voids in the bulkhead wall toe, the low dock toe, and the breakwater would be filled for structural integrity, and repairs would be made to the pier dock. -

Part I. an Annotated Checklist of Extant Brachyuran Crabs of the World
THE RAFFLES BULLETIN OF ZOOLOGY 2008 17: 1–286 Date of Publication: 31 Jan.2008 © National University of Singapore SYSTEMA BRACHYURORUM: PART I. AN ANNOTATED CHECKLIST OF EXTANT BRACHYURAN CRABS OF THE WORLD Peter K. L. Ng Raffles Museum of Biodiversity Research, Department of Biological Sciences, National University of Singapore, Kent Ridge, Singapore 119260, Republic of Singapore Email: [email protected] Danièle Guinot Muséum national d'Histoire naturelle, Département Milieux et peuplements aquatiques, 61 rue Buffon, 75005 Paris, France Email: [email protected] Peter J. F. Davie Queensland Museum, PO Box 3300, South Brisbane, Queensland, Australia Email: [email protected] ABSTRACT. – An annotated checklist of the extant brachyuran crabs of the world is presented for the first time. Over 10,500 names are treated including 6,793 valid species and subspecies (with 1,907 primary synonyms), 1,271 genera and subgenera (with 393 primary synonyms), 93 families and 38 superfamilies. Nomenclatural and taxonomic problems are reviewed in detail, and many resolved. Detailed notes and references are provided where necessary. The constitution of a large number of families and superfamilies is discussed in detail, with the positions of some taxa rearranged in an attempt to form a stable base for future taxonomic studies. This is the first time the nomenclature of any large group of decapod crustaceans has been examined in such detail. KEY WORDS. – Annotated checklist, crabs of the world, Brachyura, systematics, nomenclature. CONTENTS Preamble .................................................................................. 3 Family Cymonomidae .......................................... 32 Caveats and acknowledgements ............................................... 5 Family Phyllotymolinidae .................................... 32 Introduction .............................................................................. 6 Superfamily DROMIOIDEA ..................................... 33 The higher classification of the Brachyura ........................ -

ARQUIPELAGO Life and Marine Sciences
ARQUIPELAGO Life and Marine Sciences OPEN ACCESS ISSN 0870-4704 / e-ISSN 2182-9799 SCOPE ARQUIPELAGO - Life and Marine Sciences, publishes annually original scientific articles, short communications and reviews on the terrestrial and marine environment of Atlantic oceanic islands and seamounts. PUBLISHER University of the Azores Rua da Mãe de Deus, 58 PT – 9500-321 Ponta Delgada, Azores, Portugal. EDITOR IN CHIEF Helen Rost Martins Department of Oceanography and Fisheries / Faculty of Science and Technology University of the Azores Phone: + 351 292 200 400 / 428 E-mail: [email protected] TECHNICAL EDITOR Paula C.M. Lourinho Phone: + 351 292 200 400 / 454 E-mail: [email protected] INTERNET RESOURCES http://www.okeanos.pt/arquipelago FINANCIAL SUPPORT Okeanos-UAc – Apoio Func. e Gest. de centros I&D: 2019-DRCT-medida 1.1.a; SRMCT/GRA EDITORIAL BOARD José M.N. Azevedo, Faculty of Science and Technology, University of the Azores, Ponta Delgada, Azores; Paulo A.V. Borges, Azorean Biodiversity Group, University of the Azores, Angra do Heroísmo, Azores; João M.A. Gonçalves, Faculty of Science and Technology, University of the Azores, Horta, Azores; Louise Allcock, National University of Ireland, Galway, Ireland; Joël Bried, Cabinet vétérinaire, Biarritz, France; João Canning Clode, MARE - Marine and Environmental Sciences Centre, ARDITI, Madeira; Martin A. Collins, British Antarctic Survey, Cambridge, UK; Charles H.J.M. Fransen, Naturalis Biodiversity Center, Leiden, Netherlands, Suzanne Fredericq, Louisiana University at Lafayette, Louisiana, USA; Tony Pitcher, University of British Colombia Fisheries Center, Vancouver, Canada; Hanno Schaefer, Munich Technical University, Munich, Germany. Indexed in: Web of Science Master Journal List Cover design: Emmanuel Arand Arquipelago - Life and Marine Sciences ISSN: 0873-4704 Bryophytes of Azorean parks and gardens (I): “Reserva Florestal de Recreio do Pinhal da Paz” - São Miguel Island CLARA POLAINO-MARTIN, ROSALINA GABRIEL, PAULO A.V. -

Table 1 Comprehensive International Points List
Table 1 Comprehensive International Points List FCC ITU-T Country Region Dialing FIPS Comments, including other 1 Code Plan Code names commonly used Abu Dhabi 5 971 TC include with United Arab Emirates Aden 5 967 YE include with Yemen Admiralty Islands 7 675 PP include with Papua New Guinea (Bismarck Arch'p'go.) Afars and Assas 1 253 DJ Report as 'Djibouti' Afghanistan 2 93 AF Ajman 5 971 TC include with United Arab Emirates Akrotiri Sovereign Base Area 9 44 AX include with United Kingdom Al Fujayrah 5 971 TC include with United Arab Emirates Aland 9 358 FI Report as 'Finland' Albania 4 355 AL Alderney 9 44 GK Guernsey (Channel Islands) Algeria 1 213 AG Almahrah 5 967 YE include with Yemen Andaman Islands 2 91 IN include with India Andorra 9 376 AN Anegada Islands 3 1 VI include with Virgin Islands, British Angola 1 244 AO Anguilla 3 1 AV Dependent territory of United Kingdom Antarctica 10 672 AY Includes Scott & Casey U.S. bases Antigua 3 1 AC Report as 'Antigua and Barbuda' Antigua and Barbuda 3 1 AC Antipodes Islands 7 64 NZ include with New Zealand Argentina 8 54 AR Armenia 4 374 AM Aruba 3 297 AA Part of the Netherlands realm Ascension Island 1 247 SH Ashmore and Cartier Islands 7 61 AT include with Australia Atafu Atoll 7 690 TL include with New Zealand (Tokelau) Auckland Islands 7 64 NZ include with New Zealand Australia 7 61 AS Australian External Territories 7 672 AS include with Australia Austria 9 43 AU Azerbaijan 4 994 AJ Azores 9 351 PO include with Portugal Bahamas, The 3 1 BF Bahrain 5 973 BA Balearic Islands 9 34 SP include -

Introducing the Blue Belt Programme
Introducing–– the Blue Belt Programme 2017 - Printed and published on recycled paper Introduction The Blue Belt Programme supports delivery of the U.K. Government’s manifesto commitment to provide long term protection of over four million square kilometres of marine environment across the UK Overseas Territories. It provides £20 million over four years (2016 to 2020) to: • Improve scientific understanding of the marine environment; • Develop and implement evidence-based, tailored marine management strategies including surveillance and enforcement; and • Ensure management is sustainable and long term. The UK and the UK Overseas Territories are custodians to the fifth-largest marine estate in the world. These territories and their waters are home to globally significant biodiversity, from vast penguin colonies in the South Atlantic to tropical rainforests in the Caribbean. Some of their species and habitats are found nowhere else on earth. • 94% of British endemic species are found within the territories. • 85% of the Critically Endangered species (for which the UK Government is responsible) are within the territories. The Blue Belt Programme is initially focused on seven islands and archipelagos: British Indian Ocean Territory, South Georgia and the South Sandwich Islands, British Antarctic Territory, Pitcairn, St Helena, Ascension Island and Tristan da Cunha. The programme is being delivered in partnership between the Centre for Environment, Fisheries and Aquaculture Science (Cefas) and the Marine Management Organisation (MMO). We are also working closely with the UK Overseas Territories on behalf of the Foreign and Commonwealth Office (FCO) and the Department for Environment, Food and Rural Affairs (Defra). The Blue Belt Programme is also committed to working with NGOs, academics and external stakeholders, to ensure we have access to world-leading research and experience as we move forward with the delivery of the programme.