Beamex White Paper
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

11 Fluid Statics
CHAPTER 11 | FLUID STATICS 357 11 FLUID STATICS Figure 11.1 The fluid essential to all life has a beauty of its own. It also helps support the weight of this swimmer. (credit: Terren, Wikimedia Commons) Learning Objectives 11.1. What Is a Fluid? • State the common phases of matter. • Explain the physical characteristics of solids, liquids, and gases. • Describe the arrangement of atoms in solids, liquids, and gases. 11.2. Density • Define density. • Calculate the mass of a reservoir from its density. • Compare and contrast the densities of various substances. 11.3. Pressure • Define pressure. • Explain the relationship between pressure and force. • Calculate force given pressure and area. 11.4. Variation of Pressure with Depth in a Fluid • Define pressure in terms of weight. • Explain the variation of pressure with depth in a fluid. • Calculate density given pressure and altitude. 11.5. Pascal’s Principle • Define pressure. • State Pascal’s principle. • Understand applications of Pascal’s principle. • Derive relationships between forces in a hydraulic system. 11.6. Gauge Pressure, Absolute Pressure, and Pressure Measurement • Define gauge pressure and absolute pressure. • Understand the working of aneroid and open-tube barometers. 11.7. Archimedes’ Principle • Define buoyant force. • State Archimedes’ principle. • Understand why objects float or sink. • Understand the relationship between density and Archimedes’ principle. 11.8. Cohesion and Adhesion in Liquids: Surface Tension and Capillary Action • Understand cohesive and adhesive forces. • Define surface tension. • Understand capillary action. 11.9. Pressures in the Body • Explain the concept of pressure the in human body. • Explain systolic and diastolic blood pressures. • Describe pressures in the eye, lungs, spinal column, bladder, and skeletal system. -

A Concurrent PASCAL Compiler for Minicomputers
512 Appendix A DIFFERENCES BETWEEN UCSD'S PASCAL AND STANDARD PASCAL The PASCAL language used in this book contains most of the features described by K. Jensen and N. Wirth in PASCAL User Manual and Report, Springer Verlag, 1975. We refer to the PASCAL defined by Jensen and Wirth as "Standard" PASCAL, because of its widespread acceptance even though no international standard for the language has yet been established. The PASCAL used in this book has been implemented at University of California San Diego (UCSD) in a complete software system for use on a variety of small stand-alone microcomputers. This will be referred to as "UCSD PASCAL", which differs from the standard by a small number of omissions, a very small number of alterations, and several extensions. This appendix provides a very brief summary Of these differences. Only the PASCAL constructs used within this book will be mentioned herein. Documents are available from the author's group at UCSD describing UCSD PASCAL in detail. 1. CASE Statements Jensen & Wirth state that if there is no label equal to the value of the case statement selector, then the result of the case statement is undefined. UCSD PASCAL treats this situation by leaving the case statement normally with no action being taken. 2. Comments In UCSD PASCAL, a comment appears between the delimiting symbols "(*" and "*)". If the opening delimiter is followed immediately by a dollar sign, as in "(*$", then the remainder of the comment is treated as a directive to the compiler. The only compiler directive mentioned in this book is (*$G+*), which tells the compiler to allow the use of GOTO statements. -

Pressure, Its Units of Measure and Pressure References
_______________ White Paper Pressure, Its Units of Measure and Pressure References Viatran Phone: 1‐716‐629‐3800 3829 Forest Parkway Fax: 1‐716‐693‐9162 Suite 500 [email protected] Wheatfield, NY 14120 www.viatran.com This technical note is a summary reference on the nature of pressure, some common units of measure and pressure references. Read this and you won’t have to wait for the movie! PRESSURE Gas and liquid molecules are in constant, random motion called “Brownian” motion. The average speed of these molecules increases with increasing temperature. When a gas or liquid molecule collides with a surface, momentum is imparted into the surface. If the molecule is heavy or moving fast, more momentum is imparted. All of the collisions that occur over a given area combine to result in a force. The force per unit area defines the pressure of the gas or liquid. If we add more gas or liquid to a constant volume, then the number of collisions must increase, and therefore pressure must increase. If the gas inside the chamber is heated, the gas molecules will speed up, impact with more momentum and pressure increases. Pressure and temperature therefore are related (see table at right). The lowest pressure possible in nature occurs when there are no molecules at all. At this point, no collisions exist. This condition is known as a pure vacuum, or the absence of all matter. It is also possible to cool a liquid or gas until all molecular motion ceases. This extremely cold temperature is called “absolute zero”, which is -459.4° F. -
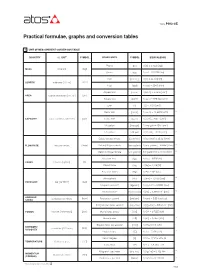
Practical Formulae, Graphs and Conversion Tables
Table P003-4/E Practical formulae, graphs and conversion tables 1 UNIT OF MEASUREMENT CONVERSION TABLE QUANTITY S.I. UNIT SYMBOL OTHER UNITS SYMBOL EQUIVALENCE Pound [lb] 1 [lb] = 0,4536 [kg] kilogram [kg] MASS Ounce [oz] 1 [oz] = 0,02335 [kg] Inch [in] or [”] 1 [in] = 25,40 [mm] millimeter [10-3 m] [mm] LENGTH Foot [foot] 1 [foot] = 304,8 [mm] Square inch [sq in] 1 [sq in] = 6,4516 [cm2] -4 2 [cm2] AREA square centimeter [10 m ] Square foot [sq ft] 1 [sq ft] = 929,034 [cm2] Liter [l] 1 [l] = 1000 [cm3] Cubic inch [cu in] 1 [cu in] = 16,3870 [cm3] * cubic centimeter [10-6 m3] [cm3] Cubic foot [cu ft] 1 [cu ft] = 28317 [cm3] CAPACITY UK gallon [Imp gal] 1 [Imp gal] = 4546 [cm3] US gallon [US gal] 1 [US gal] = 3785 [cm3] * Cubic foot per minute [cu ft/min] 1 [cu ft/min] = 28,32 [l/min] liter per minute [l/min] Gallon (UK) per minute [Imp gal/min] 1 [Imp gal/min] = 4,5456 [l/min] * FLOW RATE Gallon (US) per minute [US gal/min] [US gal/min] = 3,7848 [l/min] * Kilogram force [kgf] 1 [kgf] = 9,806 [N] Newton [kgm/s2] [N] FORCE Pound force [lbf] 1 [lbf] = 4,448 [N] Pascal [1 N/m2] [Pa] 1 [Pa] = 10-5 [bar] Atmosphere [atm] 1 [atm] = 1,0132 [bar] * bar [105 N/m2] [bar] PRESSURE 2 2 2 Kilogram force/cm [kgf/cm ] 1 [kgf/cm ] = 0,9806 [bar] 2 2 -2 Pound force/in [lbf /in ] or [psi] 1 [psi] = 6,8948•10 [bar] * ANGULAR revolution per minute [rpm] Radian per second [rad/sec] 1 [rpm] = 9,55 [rad/sec] SPEED -3 Kilogram per meter second [kgf •m/s] 1 [kgf •m/s] = 9,803•10 [kW] kilowatt [1000 Nm/s] [kW] Metric horse power [CV] 1 [CV] = 0,7355 [kW] POWER -

American and BRITISH UNITS of Measurement to SI UNITS
AMERICAN AND BRITISH UNITS OF MEASUREMENT TO SI UNITS UNIT & ABBREVIATION SI UNITS CONVERSION* UNIT & ABBREVIATION SI UNITS CONVERSION* UNITS OF LENGTH UNITS OF MASS 1 inch = 40 lines in 2.54 cm 0.393701 1 grain gr 64.7989 mg 0.0154324 1 mil 25.4 µm 0.03937 1 dram dr 1.77185 g 0.564383 1 line 0.635 mm 1.57480 1 ounce = 16 drams oz 28.3495 g 0.0352739 1 foot = 12 in = 3 hands ft 30.48 cm 0.0328084 1 pound = 16 oz lb 0.453592 kg 2.204622 1 yard = 3 feet = 4 spans yd 0.9144 m 1.09361 1 quarter = 28 lb 12.7006 kg 0.078737 1 fathom = 2 yd fath 1.8288 m 0.546807 1 hundredweight = 112 lb cwt 50.8024 kg 0.0196841 1 rod (perch, pole) rd 5.0292 m 0.198839 1 long hundredweight l cwt 50.8024 kg 0.0196841 1 chain = 100 links ch 20.1168 m 0.0497097 1 short hundredweight sh cwt 45.3592 kg 0.0220462 1 furlong = 220 yd fur 0.201168 km 4.97097 1 ton = 1 long ton tn, l tn 1.016047 t 0.984206 1 mile (Land Mile) mi 1.60934 km 0.62137 1 short ton = 2000 lb sh tn 0.907185 t 1.102311 1 nautical mile (intl.) n mi, NM 1.852 km 0.539957 1 knot (Knoten) kn 1.852 km/h 0.539957 UNITS OF FORCE 1 pound-weight lb wt 4.448221 N 0.2248089 UNITS OF AREA 1 pound-force LB, lbf 4.448221 N 0.2248089 1 square inch sq in 6.4516 cm2 0.155000 1 poundal pdl 0.138255 N 7.23301 1 circular inch 5.0671 cm2 0.197352 1 kilogram-force kgf, kgp 9.80665 N 0.1019716 1 square foot = 144 sq in sq ft 929.03 cm2 1.0764 x 10-4 1 short ton-weight sh tn wt 8.896444 kN 0.1124045 1 square yard = 9 sq ft sq yd 0.83613 m2 1.19599 1 long ton-weight l tn wt 9.964015 kN 0.1003611 1 acre = 4 roods 4046.8 -

Pressure Measuring Instruments
testo-312-2-3-4-P01 21.08.2012 08:49 Seite 1 We measure it. Pressure measuring instruments For gas and water installers testo 312-2 HPA testo 312-3 testo 312-4 BAR °C www.testo.com testo-312-2-3-4-P02 23.11.2011 14:37 Seite 2 testo 312-2 / testo 312-3 We measure it. Pressure meters for gas and water fitters Use the testo 312-2 fine pressure measuring instrument to testo 312-2 check flue gas draught, differential pressure in the combustion chamber compared with ambient pressure testo 312-2, fine pressure measuring or gas flow pressure with high instrument up to 40/200 hPa, DVGW approval, incl. alarm display, battery and resolution. Fine pressures with a resolution of 0.01 hPa can calibration protocol be measured in the range from 0 to 40 hPa. Part no. 0632 0313 DVGW approval according to TRGI for pressure settings and pressure tests on a gas boiler. • Switchable precision range with a high resolution • Alarm display when user-defined limit values are • Compensation of measurement fluctuations caused by exceeded temperature • Clear display with time The versatile pressure measuring instrument testo 312-3 testo 312-3 supports load and gas-rightness tests on gas and water pipelines up to 6000 hPa (6 bar) quickly and reliably. testo 312-3 versatile pressure meter up to Everything you need to inspect gas and water pipe 300/600 hPa, DVGW approval, incl. alarm display, battery and calibration protocol installations: with the electronic pressure measuring instrument testo 312-3, pressure- and gas-tightness can be tested. -

Surface Tension Measurement." Copyright 2000 CRC Press LLC
David B. Thiessen, et. al.. "Surface Tension Measurement." Copyright 2000 CRC Press LLC. <http://www.engnetbase.com>. Surface Tension Measurement 31.1 Mechanics of Fluid Surfaces 31.2 Standard Methods and Instrumentation Capillary Rise Method • Wilhelmy Plate and du Noüy Ring Methods • Maximum Bubble Pressure Method • Pendant Drop and Sessile Drop Methods • Drop Weight or Volume David B. Thiessen Method • Spinning Drop Method California Institute of Technology 31.3 Specialized Methods Dynamic Surface Tension • Surface Viscoelasticity • Kin F. Man Measurements at Extremes of Temperature and Pressure • California Institute of Technology Interfacial Tension The effect of surface tension is observed in many everyday situations. For example, a slowly leaking faucet drips because the force of surface tension allows the water to cling to it until a sufficient mass of water is accumulated to break free. Surface tension can cause a steel needle to “float” on the surface of water although its density is much higher than that of water. The surface of a liquid can be thought of as having a skin that is under tension. A liquid droplet is somewhat analogous to a balloon filled with air. The elastic skin of the balloon contains the air inside at a slightly higher pressure than the surrounding air. The surface of a liquid droplet likewise contains the liquid in the droplet at a pressure that is slightly higher than ambient. A clean liquid surface, however, is not elastic like a rubber skin. The tension in a piece of rubber increases as it is stretched and will eventually rupture. A clean liquid surface can be expanded indefinitely without changing the surface tension. -

Lecture # 04 Pressure Measurement Techniques and Instrumentation
AerE 344 class notes LectureLecture ## 0404 PressurePressure MeasurementMeasurement TechniquesTechniques andand InstrumentationInstrumentation Hui Hu Department of Aerospace Engineering, Iowa State University Ames, Iowa 50011, U.S.A Copyright © by Dr. Hui Hu @ Iowa State University. All Rights Reserved! MeasurementMeasurement TechniquesTechniques forfor ThermalThermal--FluidsFluids StudiesStudies Velocity, temperature, density (concentration), etc.. • Pitot probe • hotwire, hot film Intrusive • thermocouples techniques • etc ... Thermal-Fluids measurement techniques • Laser Doppler Velocimetry (LDV) particle-based • Planar Doppler Velocimetry (PDV) techniques • Particle Image Velocimetry (PIV) • etc… Non-intrusive techniques • Laser Induced Fluorescence (LIF) • Molecular Tagging Velocimetry (MTV) molecule-based • Molecular Tagging Therometry (MTT) techniques • Pressure Sensitive Paint (PSP) • Temperature Sensitive Paint (TSP) • Quantum Dot Imaging • etc … Copyright © by Dr. Hui Hu @ Iowa State University. All Rights Reserved! Pressure measurements • Pressure is defined as the amount of force that presses on a certain area. – The pressure on the surface will increase if you make the force on an area bigger. – Making the area smaller and keeping the force the same also increase the pressure. – Pressure is a scalar F dF P = n = n A dA nˆ dFn dA τˆ Copyright © by Dr. Hui Hu @ Iowa State University. All Rights Reserved! Pressure measurements Pgauge = Pabsolute − Pamb Manometer Copyright © by Dr. Hui Hu @ Iowa State University. All Rights Reserved! -

Guide for the Use of the International System of Units (SI)
Guide for the Use of the International System of Units (SI) m kg s cd SI mol K A NIST Special Publication 811 2008 Edition Ambler Thompson and Barry N. Taylor NIST Special Publication 811 2008 Edition Guide for the Use of the International System of Units (SI) Ambler Thompson Technology Services and Barry N. Taylor Physics Laboratory National Institute of Standards and Technology Gaithersburg, MD 20899 (Supersedes NIST Special Publication 811, 1995 Edition, April 1995) March 2008 U.S. Department of Commerce Carlos M. Gutierrez, Secretary National Institute of Standards and Technology James M. Turner, Acting Director National Institute of Standards and Technology Special Publication 811, 2008 Edition (Supersedes NIST Special Publication 811, April 1995 Edition) Natl. Inst. Stand. Technol. Spec. Publ. 811, 2008 Ed., 85 pages (March 2008; 2nd printing November 2008) CODEN: NSPUE3 Note on 2nd printing: This 2nd printing dated November 2008 of NIST SP811 corrects a number of minor typographical errors present in the 1st printing dated March 2008. Guide for the Use of the International System of Units (SI) Preface The International System of Units, universally abbreviated SI (from the French Le Système International d’Unités), is the modern metric system of measurement. Long the dominant measurement system used in science, the SI is becoming the dominant measurement system used in international commerce. The Omnibus Trade and Competitiveness Act of August 1988 [Public Law (PL) 100-418] changed the name of the National Bureau of Standards (NBS) to the National Institute of Standards and Technology (NIST) and gave to NIST the added task of helping U.S. -

Multidisciplinary Design Project Engineering Dictionary Version 0.0.2
Multidisciplinary Design Project Engineering Dictionary Version 0.0.2 February 15, 2006 . DRAFT Cambridge-MIT Institute Multidisciplinary Design Project This Dictionary/Glossary of Engineering terms has been compiled to compliment the work developed as part of the Multi-disciplinary Design Project (MDP), which is a programme to develop teaching material and kits to aid the running of mechtronics projects in Universities and Schools. The project is being carried out with support from the Cambridge-MIT Institute undergraduate teaching programe. For more information about the project please visit the MDP website at http://www-mdp.eng.cam.ac.uk or contact Dr. Peter Long Prof. Alex Slocum Cambridge University Engineering Department Massachusetts Institute of Technology Trumpington Street, 77 Massachusetts Ave. Cambridge. Cambridge MA 02139-4307 CB2 1PZ. USA e-mail: [email protected] e-mail: [email protected] tel: +44 (0) 1223 332779 tel: +1 617 253 0012 For information about the CMI initiative please see Cambridge-MIT Institute website :- http://www.cambridge-mit.org CMI CMI, University of Cambridge Massachusetts Institute of Technology 10 Miller’s Yard, 77 Massachusetts Ave. Mill Lane, Cambridge MA 02139-4307 Cambridge. CB2 1RQ. USA tel: +44 (0) 1223 327207 tel. +1 617 253 7732 fax: +44 (0) 1223 765891 fax. +1 617 258 8539 . DRAFT 2 CMI-MDP Programme 1 Introduction This dictionary/glossary has not been developed as a definative work but as a useful reference book for engi- neering students to search when looking for the meaning of a word/phrase. It has been compiled from a number of existing glossaries together with a number of local additions. -

Pressure Measurement Explained
Pressure measurement explained Rev A1, May 25th, 2018 Sens4Knowledge Sens4 A/S – Nordre Strandvej 119 G – 3150 Hellebaek – Denmark Phone: +45 8844 7044 – Email: [email protected] www.sens4.com Sens4Knowledge Pressure measurement explained Introduction Pressure is defined as the force per area that can be exerted by a liquid, gas or vapor etc. on a given surface. The applied pressure can be measured as absolute, gauge or differential pressure. Pressure can be measured directly by measurement of the applied force or indirectly, e.g. by the measurement of the gas properties. Examples of indirect measurement techniques that are using gas properties are thermal conductivity or ionization of gas molecules. Before mechanical manometers and electronic diaphragm pressure sensors were invented, pressure was measured by liquid manometers with mercury or water. Pressure standards In physical science the symbol for pressure is p and the SI (abbreviation from French Le Système. International d'Unités) unit for measuring pressure is pascal (symbol: Pa). One pascal is the force of one Newton per square meter acting perpendicular on a surface. Other commonly used pressure units for stating the pressure level are psi (pounds per square inch), torr and bar. Use of pressure units have regional and applicational preference: psi is commonly used in the United States, while bar the preferred unit of measure in Europe. In the industrial vacuum community, the preferred pressure unit is torr in the United States, mbar in Europe and pascal in Asia. Unit conversion Pa bar psi torr atm 1 Pa = 1 1×10-5 1.45038×10-4 7.50062×10-3 9.86923×10-6 1 bar = 100,000 1 14.5038 750.062 0.986923 1 psi = 6,894.76 6.89476×10-2 1 51.7149 6.80460×10-2 1 torr = 133.322 1.33322×10-3 1.933768×10-2 1 1.31579×10-3 1 atm (standard) = 1013.25 1.01325 14.6959 760.000 1 According to the International Organization for Standardization the standard ISO 2533:1975 defines the standard atmospheric pressure of 101,325 Pa (1 atm, 1013.25 mbar or 14.6959 psi). -

Common Units
Civil Engineering Hydraulics Pressure and Fluid Statics Leonard: It wouldn't kill us to meet new people. Sheldon: For the record, it could kill us to meet new people. Common Units ¢ In order to be able to discuss and analyze fluid problems we need to be able to understand some fundamental terms commonly used 2 Pressure 1 Common Units ¢ The most used term in hydraulics and fluid mechanics is probably pressure ¢ Pressure is defined as the normal force exerted by a fluid per unit of area l The important part of that definition is the normal (perpendicular) to the unit of area 3 Pressure Common Units ¢ The Pascal is a very small unit of pressure so it is most often encountered with a prefix to allow the numerical values to be easy to display ¢ Common prefixes are the Kilopascal (kPa=103Pa), the Megapascal (MPa=106Pa), and sometimes the Gigapascal (GPa=109Pa) 4 Pressure 2 Common Units ¢ A bar is defined as 105 Pa so a millibar (mbar) is defined as 10-3 bar so the millibar is 102 Pa The word bar finds its origin in the Greek word báros, meaning weight. 5 Pressure Common Units ¢ Standard atmospheric pressure or "the standard atmosphere" (1 atm) is defined as 101.325 kilopascals (kPa). 6 Pressure 3 Common Units ¢ This "standard pressure" is a purely arbitrary representative value for pressure at sea level, and real atmospheric pressures vary from place to place and moment to moment everywhere in the world. 7 Pressure Common Units ¢ Pressure is usually given in reference to some datum l Absolute pressure is given in reference to a system with