A Functional MRI Study of the Human Prefrontal Cortex During A
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cortical Modulation of Pupillary Function: Systematic Review
Cortical modulation of pupillary function: systematic review Costanza Peinkhofer1,2,*, Gitte M. Knudsen1,3,4, Rita Moretti2,5 and Daniel Kondziella1,4,6,* 1 Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Copenhagen, Denmark 2 Medical Faculty, University of Trieste, Trieste, Italy 3 Neurobiology Research Unit, Rigshospitalet, Copenhagen University Hospital, Copenhagen, Denmark 4 Faculty of Health and Medical Science, University of Copenhagen, Copenhagen, Denmark 5 Department of Medical, Surgical and Health Sciences, Neurological Unit, Trieste University Hospital, Cattinara, Trieste, Italy 6 Department of Neuroscience, Norwegian University of Technology and Science, Trondheim, Norway * These authors contributed equally to this work. ABSTRACT Background. The pupillary light reflex is the main mechanism that regulates the pupillary diameter; it is controlled by the autonomic system and mediated by subcortical pathways. In addition, cognitive and emotional processes influence pupillary function due to input from cortical innervation, but the exact circuits remain poorly understood. We performed a systematic review to evaluate the mechanisms behind pupillary changes associated with cognitive efforts and processing of emotions and to investigate the cerebral areas involved in cortical modulation of the pupillary light reflex. Methodology. We searched multiple databases until November 2018 for studies on cortical modulation of pupillary function in humans and non-human primates. Of 8,809 papers screened, 258 studies -
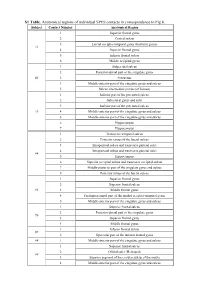
S1 Table. Anatomical Regions of Individual SPES Contacts in Correspondence to Fig 8
S1 Table. Anatomical regions of individual SPES contacts in correspondence to Fig 8. Subject Contact Number Anatomical Region 1 Superior frontal gyrus 2 Central sulcus 3 Lateral occipito-temporal gyrus (fusiform gyrus) #1 4 Superior frontal gyrus 5 Inferior frontal sulcus 6 Middle occipital gyrus 1 Subparietal sulcus 2 Posterior-dorsal part of the cingulate gyrus #2 3 Precuneus 4 Middle-anterior part of the cingulate gyrus and sulcus 5 Sulcus intermedius primus (of Jensen) 1 Inferior part of the precentral sulcus 2 Subcentral gyrus and sulci 3 Inferior part of the precentral sulcus #3 4 Middle-anterior part of the cingulate gyrus and sulcus 5 Middle-anterior part of the cingulate gyrus and sulcus 6 Hippocampus 7 Hippocampus 1 Transverse temporal sulcus 2 Posterior ramus of the lateral sulcus 3 Intraparietal sulcus and transverse parietal sulci 4 Intraparietal sulcus and transverse parietal sulci #4 5 Hippocampus 6 Superior occipital sulcus and transverse occipital sulcus 7 Middle-posterior part of the cingulate gyrus and sulcus 8 Posterior ramus of the lateral sulcus 1 Superior frontal gyrus 2 Superior frontal sulcus #5 3 Middle frontal gyrus 4 Parahippocampal part of the medial occipito-temporal gyrus 5 Middle-anterior part of the cingulate gyrus and sulcus 1 Superior frontal sulcus 2 Posterior-dorsal part of the cingulate gyrus #6 3 Superior frontal gyrus 4 Middle frontal gyrus 1 Inferior frontal sulcus #7 2 Opercular part of the inferior frontal gyrus #8 1 Middle-anterior part of the cingulate gyrus and sulcus 1 Superior frontal sulcus 2 Orbital sulci (H-shaped) #9 3 Superior segment of the circular sulcus of the insula 4 Middle-anterior part of the cingulate gyrus and sulcus . -

Connectivity of BA46 Involvement in the Executive Control of Language
Alfredo Ardila, Byron Bernal and Monica Rosselli Psicothema 2016, Vol. 28, No. 1, 26-31 ISSN 0214 - 9915 CODEN PSOTEG Copyright © 2016 Psicothema doi: 10.7334/psicothema2015.174 www.psicothema.com Connectivity of BA46 involvement in the executive control of language Alfredo Ardila1, Byron Bernal2 and Monica Rosselli3 1 Florida International University, 2 Miami Children’s Hospital and 3 Florida Atlantic University Abstract Resumen Background: Understanding the functions of different brain areas has Estudio de la conectividad del AB46 en el control ejecutivo del lenguaje. represented a major endeavor of contemporary neurosciences. Modern Antecedentes: la comprensión de las funciones de diferentes áreas neuroimaging developments suggest cognitive functions are associated cerebrales representa una de las mayores empresas de las neurociencias with networks rather than with specifi c areas. Objectives. The purpose contemporáneas. Los estudios contemporáneos con neuroimágenes of this paper was to analyze the connectivity of Brodmann area (BA) 46 sugieren que las funciones cognitivas se asocian con redes más que con (anterior middle frontal gyrus) in relation to language. Methods: A meta- áreas específi cas. El propósito de este estudio fue analizar la conectividad analysis was conducted to assess the language network in which BA46 is del área de Brodmann 46 (BA46) (circunvolución frontal media anterior) involved. The DataBase of Brainmap was used; 19 papers corresponding con relación al lenguaje. Método: se llevó a cabo un meta-análisis para to 60 experimental conditions with a total of 245 subjects were included. determinar el circuito o red lingüística en la cual participa BA46. Se utilizó Results: Our results suggest the core network of BA46. -

Dorsolateral Prefrontal Cortex-Based Control with an Implanted Brain–Computer Interface
www.nature.com/scientificreports OPEN Dorsolateral prefrontal cortex‑based control with an implanted brain–computer interface Sacha Leinders 1, Mariska J. Vansteensel 1, Mariana P. Branco 1, Zac V. Freudenburg1, Elmar G. M. Pels1, Benny Van der Vijgh1, Martine J. E. Van Zandvoort2, Nicolas F. Ramsey1* & Erik J. Aarnoutse 1 The objective of this study was to test the feasibility of using the dorsolateral prefrontal cortex as a signal source for brain–computer interface control in people with severe motor impairment. We implanted two individuals with locked‑in syndrome with a chronic brain–computer interface designed to restore independent communication. The implanted system (Utrecht NeuroProsthesis) included electrode strips placed subdurally over the dorsolateral prefrontal cortex. In both participants, counting backwards activated the dorsolateral prefrontal cortex consistently over the course of 47 and 22months, respectively. Moreover, both participants were able to use this signal to control a cursor in one dimension, with average accuracy scores of 78 ± 9% (standard deviation) and 71 ± 11% (chance level: 50%), respectively. Brain– computer interface control based on dorsolateral prefrontal cortex activity is feasible in people with locked‑in syndrome and may become of relevance for those unable to use sensorimotor signals for control. Locked-in syndrome (LIS) is characterized by intact cognition and (almost) complete loss of voluntary movement1. Brain–computer interface (BCI) systems allow computer control with brain activity and can there- fore provide an alternative communication channel to people with LIS. BCI systems depending on self-initiated modulation of brain activity generally measure from sensorimotor areas, e.g.2–9. However, not all people with LIS may be able to reliably modulate sensorimotor activity, due to for instance atrophy secondary to neurode- generative disease10,11, damage afer stroke or injury, or atypical neural activity 12. -

Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans Ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai
Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai To cite this version: Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai. Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex. Frontiers in Neuroanatomy, Frontiers, 2018, 12, pp.93. 10.3389/fnana.2018.00093. hal-01929541 HAL Id: hal-01929541 https://hal.archives-ouvertes.fr/hal-01929541 Submitted on 21 Nov 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. REVIEW published: 19 November 2018 doi: 10.3389/fnana.2018.00093 Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans J. ten Donkelaar 1*†, Nathalie Tzourio-Mazoyer 2† and Jürgen K. Mai 3† 1 Department of Neurology, Donders Center for Medical Neuroscience, Radboud University Medical Center, Nijmegen, Netherlands, 2 IMN Institut des Maladies Neurodégénératives UMR 5293, Université de Bordeaux, Bordeaux, France, 3 Institute for Anatomy, Heinrich Heine University, Düsseldorf, Germany The gyri and sulci of the human brain were defined by pioneers such as Louis-Pierre Gratiolet and Alexander Ecker, and extensified by, among others, Dejerine (1895) and von Economo and Koskinas (1925). -

Insular Volume Reductions in Patients with Major Depressive Disorder
Insular volume reductions in patients with major depressive disorder Item Type Article Authors Mutschler, Isabella; Hänggi, Jürgen; Frei, Manuela; Lieb, Roselind; grosse Holforth, Martin; Seifritz, Erich; Spinelli, Simona Citation Mutschler, I., Hänggi, J., Frei, M., Lieb, R., grosse Holforth, M., Seifritz, E., & Spinelli, S. (2019). Insular volume reductions in patients with major depressive disorder. Neurology, Psychiatry and Brain Research, 33, 39–47. doi:10.1016/j.npbr.2019.06.002 Eprint version Post-print DOI 10.1016/j.npbr.2019.06.002 Publisher Elsevier BV Journal Neurology Psychiatry and Brain Research Rights NOTICE: this is the author’s version of a work that was accepted for publication in Neurology Psychiatry and Brain Research. Changes resulting from the publishing process, such as peer review, editing, corrections, structural formatting, and other quality control mechanisms may not be reflected in this document. Changes may have been made to this work since it was submitted for publication. A definitive version was subsequently published in Neurology Psychiatry and Brain Research, [[Volume], [Issue], (2019-06-22)] DOI: 10.1016/ j.npbr.2019.06.002 . © 2019. This manuscript version is made available under the CC-BY-NC-ND 4.0 license http:// creativecommons.org/licenses/by-nc-nd/4.0/ Download date 23/09/2021 13:26:26 Item License http://creativecommons.org/licenses/by-nc-nd/4.0/ Link to Item http://hdl.handle.net/10754/656271 Neurology, Psychiatry and Brain Research 33 (2019) 39–47 Contents lists available at ScienceDirect Neurology, -

Efficacy of Transcranial Magnetic Stimulation Targets for Depression
Efficacy of Transcranial Magnetic Stimulation Targets for Depression Is Related to Intrinsic Functional Connectivity with the Subgenual Cingulate Michael D. Fox, Randy L. Buckner, Matthew P. White, Michael D. Greicius, and Alvaro Pascual-Leone Background: Transcranial magnetic stimulation (TMS) to the left dorsolateral prefrontal cortex (DLPFC) is used clinically for the treatment of depression. However, the antidepressant mechanism remains unknown and its therapeutic efficacy remains limited. Recent data suggest that some left DLPFC targets are more effective than others; however, the reasons for this heterogeneity and how to capitalize on this information remain unclear. Methods: Intrinsic (resting state) functional magnetic resonance imaging data from 98 normal subjects were used to compute functional connectivity with various left DLPFC TMS targets employed in the literature. Differences in functional connectivity related to differences in previously reported clinical efficacy were identified. This information was translated into a connectivity-based targeting strategy to identify optimized left DLPFC TMS coordinates. Results in normal subjects were tested for reproducibility in an independent cohort of 13 patients with depression. Results: Differences in functional connectivity were related to previously reported differences in clinical efficacy across a distributed set of cortical and limbic regions. Dorsolateral prefrontal cortex TMS sites with better clinical efficacy were more negatively correlated (anticorre- lated) with the subgenual cingulate. Optimum connectivity-based stimulation coordinates were identified in Brodmann area 46. Results were reproducible in patients with depression. Conclusions: Reported antidepressant efficacy of different left DLPFC TMS sites is related to the anticorrelation of each site with the subgenual cingulate, potentially lending insight into the antidepressant mechanism of TMS and suggesting a role for intrinsically anticor- related networks in depression. -

Cortical Abnormalities in Bipolar Disorder: an MRI Analysis of 6503 Individuals from the ENIGMA Bipolar Disorder Working Group
OPEN Molecular Psychiatry (2018) 23, 932–942 www.nature.com/mp ORIGINAL ARTICLE Cortical abnormalities in bipolar disorder: an MRI analysis of 6503 individuals from the ENIGMA Bipolar Disorder Working Group DP Hibar1,2, LT Westlye3,4,5, NT Doan3,4, N Jahanshad1, JW Cheung1, CRK Ching1,6, A Versace7, AC Bilderbeck8, A Uhlmann9,10, B Mwangi11, B Krämer12, B Overs13, CB Hartberg3, C Abé14, D Dima15,16, D Grotegerd17, E Sprooten18, E Bøen19, E Jimenez20, FM Howells9, G Delvecchio21, H Temmingh9, J Starke9, JRC Almeida22, JM Goikolea20, J Houenou23,24, LM Beard25, L Rauer12, L Abramovic26, M Bonnin20, MF Ponteduro16, M Keil27, MM Rive28,NYao29,30, N Yalin31, P Najt32, PG Rosa33,34, R Redlich17, S Trost27, S Hagenaars35, SC Fears36,37, S Alonso-Lana38,39, TGM van Erp40, T Nickson35, TM Chaim-Avancini33,34, TB Meier41,42, T Elvsåshagen3,43, UK Haukvik3,44, WH Lee18, AH Schene45,46, AJ Lloyd47, AH Young31, A Nugent48, AM Dale49,50, A Pfennig51, AM McIntosh35, B Lafer33, BT Baune52, CJ Ekman14, CA Zarate48, CE Bearden53,54, C Henry23,55, C Simhandl56, C McDonald32, C Bourne8,57, DJ Stein9,10, DH Wolf25, DM Cannon32, DC Glahn29,30, DJ Veltman58, E Pomarol-Clotet38,39, E Vieta20, EJ Canales-Rodriguez38,39, FG Nery33,59, FLS Duran33,34, GF Busatto33,34, G Roberts60, GD Pearlson29,30, GM Goodwin8, H Kugel61, HC Whalley35, HG Ruhe8,28,62, JC Soares11, JM Fullerton13,63, JK Rybakowski64, J Savitz42,65, KT Chaim66,67, M Fatjó-Vilas38,39, MG Soeiro-de-Souza33, MP Boks26, MV Zanetti33,34, MCG Otaduy66,67, MS Schaufelberger33,34, M Alda68, M Ingvar14,69, -

The Prefrontal Cortex
Avens Publishing Group Inviting Innovations Open Access Review Article J Hum Anat Physiol July 2017 Volume:1, Issue:1 © All rights are reserved by Ogeturk. AvensJournal Publishing of Group Inviting Innovations Human Anatomy The Prefrontal Cortex: A Basic & Physiology Embryological, Histological, Anatomical, and Functional Ramazan Fazıl Akkoc and Murat Ogeturk* Department of Anatomy, Firat University, Turkey Guideline *Address for Correspondence Murat Ogeturk, Firat University, Faculty of Medicine, 23119 Elazig, Turkey, Tel: +90-424-2370000 (ext: 4654); Fax: +90-424-2379138; Keywords: Prefrontal cortex; Working memory; Frontal lobe E-Mail: [email protected] Abstract Submission: 24 May, 2017 Accepted: 11 July, 2017 The prefrontal cortex (PFC) unites, processes and controls the Published: 19 August, 2017 information coming from cortex and subcortical structures, and Copyright: © 2017 Akkoc RF. This is an open access article distributed decides and executes goal-oriented behavior. A major function of PFC under the Creative Commons Attribution License, which permits is to maintain the attention. Furthermore, it has many other functions unrestricted use, distribution, and reproduction in any medium, provided including working memory, problem solving, graciousness, memory, the original work is properly cited. and intellectuality. PFC is well developed in humans and localized to the anterior of the frontal lobe. This article presents a systematic review and detailed summary of embryology, histology, anatomy, functions and lesions of PFC. I. Lamina zonalis: Contains few Cajal horizontal cells. The axons of Martinotti cells located at deep layers, the last branches of the apical dendrites of pyramidal cells, and the last branches Introduction of the afferent nerve fibers extend to this lamina. -

Neural Correlates Underlying Change in State Self-Esteem Hiroaki Kawamichi 1,2,3, Sho K
www.nature.com/scientificreports OPEN Neural correlates underlying change in state self-esteem Hiroaki Kawamichi 1,2,3, Sho K. Sugawara2,4,5, Yuki H. Hamano2,5,6, Ryo Kitada 2,7, Eri Nakagawa2, Takanori Kochiyama8 & Norihiro Sadato 2,5 Received: 21 July 2017 State self-esteem, the momentary feeling of self-worth, functions as a sociometer involved in Accepted: 11 January 2018 maintenance of interpersonal relations. How others’ appraisal is subjectively interpreted to change Published: xx xx xxxx state self-esteem is unknown, and the neural underpinnings of this process remain to be elucidated. We hypothesized that changes in state self-esteem are represented by the mentalizing network, which is modulated by interactions with regions involved in the subjective interpretation of others’ appraisal. To test this hypothesis, we conducted task-based and resting-state fMRI. Participants were repeatedly presented with their reputations, and then rated their pleasantness and reported their state self- esteem. To evaluate the individual sensitivity of the change in state self-esteem based on pleasantness (i.e., the subjective interpretation of reputation), we calculated evaluation sensitivity as the rate of change in state self-esteem per unit pleasantness. Evaluation sensitivity varied across participants, and was positively correlated with precuneus activity evoked by reputation rating. Resting-state fMRI revealed that evaluation sensitivity was positively correlated with functional connectivity of the precuneus with areas activated by negative reputation, but negatively correlated with areas activated by positive reputation. Thus, the precuneus, as the part of the mentalizing system, serves as a gateway for translating the subjective interpretation of reputation into state self-esteem. -

An Analysis of Functional Neuroimaging Studies of Dorsolateral Prefrontal Cortical Activity in Depression
Psychiatry Research: Neuroimaging 148 (2006) 33–45 www.elsevier.com/locate/psychresns An analysis of functional neuroimaging studies of dorsolateral prefrontal cortical activity in depression Paul B. Fitzgeralda,⁎, Tom J. Oxleya, Angela R. Lairdb, Jayashri Kulkarnia, Gary F. Eganc, Zafiris J. Daskalakisd aAlfred Psychiatry Research Centre, The Alfred and Monash University Department of Psychological Medicine, Commercial Rd, Melbourne, Victoria 3004, Australia bResearch Imaging Center, The University of Texas Health Science Center, San Antonio, San Antonio, TX, USA cHoward Florey Institute, The University of Melbourne, Victoria, Australia dCentre for Addiction and Mental Health, Clarke Division, Toronto, Ontario, Canada Received 22 December 2005; received in revised form 28 March 2006; accepted 10 April 2006 Abstract Repetitive transcranial magnetic stimulation (rTMS) is currently undergoing active investigation for use in the treatment of major depression. Recent research has indicated that current methods used to localize the site of stimulation in dorsolateral prefrontal cortex (DLPFC) are significantly inaccurate. However, little information is available on which to base a choice of stimulation site. The aim of the current study was to systematically examine imaging studies in depression to attempt to identify whether there is a pattern of imaging results that suggests an optimal site of stimulation localization. We analysed all imaging studies published prior to 2005 that examined patients with major depression. Studies reporting activation in DLPFC were identified. The DLPFC regions identified in these studies were analysed using the Talairach and Rajkowska–Goldman-Rakic coordinate systems. In addition, we conducted a quantitative meta-analysis of resting studies and studies of serotonin reuptake inhibitor antidepressant treatment. -

Healthy and Abnormal Development of the Prefrontal Cortex
Developmental Neurorehabilitation, October 2009; 12(5): 279–297 Healthy and abnormal development of the prefrontal cortex MEGAN SPENCER-SMITH1,2 & VICKI ANDERSON1,2,3 1Murdoch Childrens Research Institute, Melbourne, Australia, 2University of Melbourne, Melbourne, Australia, and 3Royal Children’s Hospital, Melbourne, Australia (Received 8 October 2008; accepted 3 June 2009) Abstract Background: While many children with brain conditions present with cognitive, behavioural, emotional, academic and social impairments, other children recover with seemingly few impairments. Animal studies and preliminary child studies have identified timing of brain lesion as a key predictor in determining functional outcome following early brain lesions. Review: This research suggests that knowledge of healthy developmental processes in brain structure and function is essential for better understanding functional recovery and outcome in children with brain lesions. This review paper aims to equip researchers with current knowledge of key principles of developmental processes in brain structure and function. Timetables for development of the prefrontal cortex (PFC), a brain region particularly vulnerable to lesions due to its protracted developmental course, are examined. In addition, timetables for development of executive skills, which emerge in childhood and have a prolonged developmental course that parallels development of the PFC, are also discussed. Conclusions: Equipped with this knowledge, researchers are now in a better position to understand functional