Cortical Modulation of Pupillary Function: Systematic Review
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Functional Brain Imaging in Apraxia
OBSERVATION Functional Brain Imaging in Apraxia David A. Kareken, PhD; Frederick Unverzagt, PhD; Karen Caldemeyer, MD; Martin R. Farlow, MD; Gary D. Hutchins, PhD Background: An extensive literature describes struc- sults of the neurological examination were normal. There tural lesions in apraxia, but few studies have used func- was ideomotor apraxia of both hands (command, imita- tional neuroimaging. We used positron emission tomog- tion, and object) and buccofacial apraxia. The patient raphy (PET) to characterize relative cerebral glucose could recognize meaningful gestures performed by the metabolism in a 65-year-old, right-handed woman with examiner and discriminate between his accurate and awk- progressive decline in ability to manipulate objects, write, ward pantomime. The magnetic resonance image showed and articulate speech. moderate generalized atrophy and mild ischemic changes. Positron emission tomographic scans showed abnormal Objective: To characterize functional brain organiza- fludeoxyglucose F 18 uptake in the posterior frontal, tion in apraxia. supplementary motor, and parietal regions, the left af- fected more than the right. Focal metabolic deficit was Design and Methods: The patient underwent a neu- present in the angular gyrus, an area hypothesized to store rological examination, neuropsychological testing, mag- conceptual knowledge of skilled movement. netic resonance imaging, and fludeoxyglucose F 18 PET. The patient’s magnetic resonance image was coregis- Conclusions: Greater parietal than frontal physiologi- tered to her PET image, which was compared with the cal dysfunction and preserved gesture recognition are not PET images of 7 right-handed, healthy controls. Hemi- consistent with the theory that knowledge of limb praxis spheric regions of interest were normalized by calcrine is stored in the dominant parietal cortex. -

Talairach Atlas Labels for Functional Brain Mapping
᭜ Human Brain Mapping 10:120–131(2000) ᭜ Automated Talairach Atlas Labels For Functional Brain Mapping Jack L. Lancaster,* Marty G. Woldorff, Lawrence M. Parsons, Mario Liotti, Catarina S. Freitas, Lacy Rainey, Peter V. Kochunov, Dan Nickerson, Shawn A. Mikiten, and Peter T. Fox Research Imaging Center, University of Texas Health Science Center at San Antonio ᭜ ᭜ Abstract: An automated coordinate-based system to retrieve brain labels from the 1988 Talairach Atlas, called the Talairach Daemon (TD), was previously introduced [Lancaster et al., 1997]. In the present study, the TD system and its 3-D database of labels for the 1988 Talairach atlas were tested for labeling of functional activation foci. TD system labels were compared with author-designated labels of activation coordinates from over 250 published functional brain-mapping studies and with manual atlas-derived labels from an expert group using a subset of these activation coordinates. Automated labeling by the TD system compared well with authors’ labels, with a 70% or greater label match averaged over all locations. Author-label matching improved to greater than 90% within a search range of Ϯ5 mm for most sites. An adaptive grey matter (GM) range-search utility was evaluated using individual activations from the M1 mouth region (30 subjects, 52 sites). It provided an 87% label match to Brodmann area labels (BA4&BA 6) within a search range of Ϯ5 mm. Using the adaptive GM range search, the TD system’s overall match with authors’ labels (90%) was better than that of the expert group (80%). When used in concert with authors’ deeper knowledge of an experiment, the TD system provides consistent and comprehensive labels for brain activation foci. -

Thalamic Atrophy in Antero-Medial and Dorsal Nuclei Correlates with Six-Month Outcome After Severe Brain Injury☆
NeuroImage: Clinical 3 (2013) 396–404 Contents lists available at ScienceDirect NeuroImage: Clinical journal homepage: www.elsevier.com/locate/ynicl Thalamic atrophy in antero-medial and dorsal nuclei correlates with six-month outcome after severe brain injury☆ Evan S. Lutkenhoff a, David L. McArthur b, Xue Hua c,PaulM.Thompsonc,d, Paul M. Vespa b,e,MartinM.Montia,b,⁎ a Department of Psychology, University of California Los Angeles, Los Angeles, CA 90095, USA b Brain Injury Research Center (BIRC), Department of Neurosurgery, David Geffen School of Medicine at UCLA, Los Angeles, CA 90095, USA c Laboratory of Neuro Imaging (LONI), Department of Neurology, David Geffen School of Medicine at UCLA, Los Angeles, CA 90095, USA d Department of Psychiatry, University of California Los Angeles, Los Angeles, CA 90095, USA e Department of Neurology, David Geffen School of Medicine at University of California Los Angeles, Los Angeles, CA 90095, USA article info abstract Article history: The primary and secondary damage to neural tissue inflicted by traumatic brain injury is a leading cause of death Received 28 June 2013 and disability. The secondary processes, in particular, are of great clinical interest because of their potential Received in revised form 26 September 2013 susceptibility to intervention. We address the dynamics of tissue degeneration in cortico-subcortical circuits Accepted 27 September 2013 after severe brain injury by assessing volume change in individual thalamic nuclei over the first six-months Available online 5 October 2013 post-injury in a sample of 25 moderate to severe traumatic brain injury patients. Using tensor-based morphom- etry, we observed significant localized thalamic atrophy over the six-month period in antero-dorsal limbic nuclei Keywords: Traumatic brain injury as well as in medio-dorsal association nuclei. -

Molecular Characterization of Selectively Vulnerable Neurons in Alzheimer’S Disease
RESOURCE https://doi.org/10.1038/s41593-020-00764-7 Molecular characterization of selectively vulnerable neurons in Alzheimer’s disease Kun Leng1,2,3,4,13, Emmy Li1,2,3,13, Rana Eser 5, Antonia Piergies5, Rene Sit2, Michelle Tan2, Norma Neff 2, Song Hua Li5, Roberta Diehl Rodriguez6, Claudia Kimie Suemoto7,8, Renata Elaine Paraizo Leite7, Alexander J. Ehrenberg 5, Carlos A. Pasqualucci7, William W. Seeley5,9, Salvatore Spina5, Helmut Heinsen7,10, Lea T. Grinberg 5,7,11 ✉ and Martin Kampmann 1,2,12 ✉ Alzheimer’s disease (AD) is characterized by the selective vulnerability of specific neuronal populations, the molecular sig- natures of which are largely unknown. To identify and characterize selectively vulnerable neuronal populations, we used single-nucleus RNA sequencing to profile the caudal entorhinal cortex and the superior frontal gyrus—brain regions where neurofibrillary inclusions and neuronal loss occur early and late in AD, respectively—from postmortem brains spanning the progression of AD-type tau neurofibrillary pathology. We identified RORB as a marker of selectively vulnerable excitatory neu- rons in the entorhinal cortex and subsequently validated their depletion and selective susceptibility to neurofibrillary inclusions during disease progression using quantitative neuropathological methods. We also discovered an astrocyte subpopulation, likely representing reactive astrocytes, characterized by decreased expression of genes involved in homeostatic functions. Our characterization of selectively vulnerable neurons in AD paves the way for future mechanistic studies of selective vulnerability and potential therapeutic strategies for enhancing neuronal resilience. elective vulnerability is a fundamental feature of neurodegen- Here, we performed snRNA-seq on postmortem brain tissue erative diseases, in which different neuronal populations show from a cohort of individuals spanning the progression of AD-type a gradient of susceptibility to degeneration. -

Thalamic Atrophy in Antero-Medial and Dorsal Nuclei Correlates with Six-Month Outcome After Severe Brain Injury☆
YNICL-00167; No. of pages: 9; 4C: NeuroImage: Clinical 3 (2013) xxx–xxx Contents lists available at ScienceDirect NeuroImage: Clinical journal homepage: www.elsevier.com/locate/ynicl Thalamic atrophy in antero-medial and dorsal nuclei correlates with six-month outcome after severe brain injury☆ Evan S. Lutkenhoff a, David L. McArthur b, Xue Hua c,PaulM.Thompsonc,d, Paul M. Vespa b,e,MartinM.Montia,b,⁎ a Department of Psychology, University of California Los Angeles, Los Angeles, CA 90095, USA b Brain Injury Research Center (BIRC), Department of Neurosurgery, David Geffen School of Medicine at UCLA, Los Angeles, CA 90095, USA c Laboratory of Neuro Imaging (LONI), Department of Neurology, David Geffen School of Medicine at UCLA, Los Angeles, CA 90095, USA d Department of Psychiatry, University of California Los Angeles, Los Angeles, CA 90095, USA e Department of Neurology, David Geffen School of Medicine at University of California Los Angeles, Los Angeles, CA 90095, USA article info abstract Article history: The primary and secondary damage to neural tissue inflicted by traumatic brain injury is a leading cause of death Received 28 June 2013 and disability. The secondary processes, in particular, are of great clinical interest because of their potential Received in revised form 26 September 2013 susceptibility to intervention. We address the dynamics of tissue degeneration in cortico-subcortical circuits Accepted 27 September 2013 after severe brain injury by assessing volume change in individual thalamic nuclei over the first six-months Available online 5 October 2013 post-injury in a sample of 25 moderate to severe traumatic brain injury patients. -

Cerebral Hemisphere
Cerebral Hemisphere Derived from the embryological telencephalon Consists of : - Cerebral cortex (Layer of grey mater) highly convulated to form a complex pattern of ridges (gyri) and furrows (sulci) - Centrum semi ovale (Layer of white mater) - Basal ganglia (Nuclear masses which buried within the white matter) - Rhinencephalon • Internal capsule - Contains both ascending and descending axons • Lateral ventricle • Corpus striatum or basal ganglia caudate nucleus, putamen, and globus pallidus • Corpus callosum • Great Longitudinal fissure Corona Radiata • Fibres radiate in and out to produce a fan-like arrangement between internal capsule and cortical surface • Contains both descending and ascending axons that carry nearly all of the neural traffic from and to the cerebral cortex Corona Radiata Lobes of The Cerebral Hemisphere • Frontale lobe • Parietal lobe • Temporal lobe • Occipital lobe • Insular lobe • Limbic Cerebral Cortex Histological Structure Consist of : Archicortex and Paleocortex phylogenetically old parts of the cortex - hippocampus and other parts of the temporal lobe - Three-layered cytoarchitecture Neocortex - Six-layered cytoarchitecture Histological Structure of Cerebral Cortex • Molecular (plexiform) layer Few nerve cell bodies but many dendritic and axonal processes in synaptic interaction • OUTER granular layer Many small neurones, which establish intracortical connections • OUTER pyramidal cell layer Medium-sized neurones giving rise to association and commissural fibres • INNER granular layer The side of -

CEREBRUM (2).Pdf
CEREBRUM Dr. Jamila EL Medany Dr Essam Salama Objectives At the end of the lecture, the student should be able to: List the parts of the cerebral hemisphere (cortex, medulla, basal nuclei, lateral ventricle). Describe the subdivision of a cerebral hemisphere into lobes. List the important sulci and gyri of each lobe. Describe different types of fibers in cerebral medulla (association, projection and commissural) and give example of each type. Cerebrum Corpus callosum Largest part of the forebrain. Divided into two halves, the (cerebral hemipheres), which are separated by a deep median longitudinal Left Right fissure which lodges the falx hemisphere hemisphere cerebri. In the depth of the fissure, the hemispheres are connected by a bundle of fibers called the corpus callosum. Median longitudinal fissure Structure of Cerebrum Cortex Basal Cerebral cortex: Superficial layer ganglia of grey matter Medulla (White matter) : Deeper WM to the cortex, contains axons to and from the cells of the cortex Basal ganglia: Number of nuclear masses buried within the white matter Lateral ventricle: The cavity of hemisphere WM Lateral ventricle Surfaces(3) Superolateral Medial Inferior (tentorial) Lobes of Cerebrum The superficial layer of grey matter is highly convoluted to form a complex pattern of ridges (gyri) and grooves (sulci). This arrangement maximizes the surface area of the cerebral cortex (about 70% is hidden within the depths of sulci). • Three sulci, consistent in position, named central, lateral (sylvian) & parieto- occipital, divide each hemisphere into FOUR lobes: S g Frontal, Parietal, Temporal & Occipital (named after overlying bones) Functionally each hemisphere contains a ‘limbic lobe’ on the medial surface. -

Quantitative Assessment Suppl1.Pdf
Supporting Information Donahue et al. 10.1073/pnas.1721653115 Delineating Cortex Across Species evidence of cognitive-related function and attempted to maintain Here, we summarize evidence for the inclusion of cortical areas in PFC designations among putatively homologous areas shared either our conservative (cytoarchitecturally granular/dysgranular) with humans (e.g., areas 24, 25, and 32). or liberal (agranular but implicated in cognitive function) de- Area 24 is typically subdivided into areas 24a, 24b, and 24c. lineations of PFC. Tables S2 and S3 detail human and macaque While the Ferry et al. (2) parcellation of these areas is limited in areas, respectively, and include cytoarchitectural designations of its posterior extent due to tissue coverage limitations, the Paxinos granular (G), lightly granular (LG), dysgranular (D), or agranular et al. (3) whole-cortex parcellation allows extension of these areas (A) as reported by previous studies. Human areas correspond to to their posterior extent. We first created composite versions of those in the HCP_MMP1.0 parcellation (1), while macaque areas these two different parcellations, resulting in areas 24a′, 24b′, and are a composite of those from Ferry et al. (2), Paxinos et al. (3), 24c′. As described in Ferry et al. (2), while these regions are and Lewis and Van Essen (4) cytoarchitectural parcellations. generally considered part of their medial prefrontal network, more posterior/dorsal portions could be considered part of Human Liberal PFC Delineations. For humans, areal functional premotor cortex. For this reason, we chose to subdivide these properties were primarily drawn from Glasser et al. (1), which areas further into anterior and posterior portions, primarily describes such properties in detail. -

Supporting Information Delineating PFC Across Species Here, We
Supporting Information Delineating PFC across Species Here, we summarize evidence for inclusion of cortical areas in either our conservative (cytoarchitecturally granular/dysgranular) or liberal (agranular, but implicated in cognitive function) delineations of prefrontal cortex (PFC). Supplementary Tables 1 and 2 detail human and macaque areas, respectively, and include cytoarchitectural designations of granular (G), lightly granular (LG), dysgranular (D) or agranular (A) as reported by previous studies. Human areas correspond to those in the HCP_MMP1.0 parcellation1, while macaque areas are a composite of those from Ferry et al.2, Paxinos et al.3 and Lewis & Van Essen4 cytoarchitectural parcellations. Human Liberal PFC Delineations Decisions to include cytoarchitecturally agranular areas in our liberal PFC delineation were based on reports from previous studies implicating a given region in cognitive-related function. For humans, areal functional properties were primarily drawn from Glasser et al., 20161, which describes such properties in detail. An instructive starting example is human area 55b (26), which lies within a strip of premotor areas, but, on multiple grounds, can be considered part of liberal PFC. More specifically, area 55b is bounded by the frontal and premotor eye fields (FEF/PEF) medially and laterally, primary motor cortex posteriorly (4), ventral premotor cortex (6v) and conservative PFC areas 8C and 8Av anteriorly. Area 55b exhibits several distinctive features: (1) light myelination compared to moderately myelinated FEF/PEF and heavily myelinated primary motor cortex, (2) strong functional connectivity with a seed in language-related area PSL and (3) strong activation in contrasts of cognitive-related tasks relative to surrounding areas. Specifically, area 55b exhibited left lateralized activation in the LANGUAGE STORY & RELATIONAL-MATCH tasks and right lateralized activation in the social cognition (TOM-RANDOM) task (tasks and resulting parcellation are detailed in Glasser et al., 201631). -

Functional Specialization 2: Forebrain and Cerebral Cortex
Cerebral Cortex Lecture 4 1 Functional Specialization So Far • Neurons and Nerves • Brain – Afferent, Efferent, – Hindbrain Interneurons • Pons, Medulla, • Peripheral Nervous Cerebellum System – Midbrain • Reticular Formation – Autonomic – Forebrain Subcortical • Sympathetic, Structures Parasympathetic • Hypothalamus – Somatic • Hippocampus • Afferent, Efferent • Amygdala Pathways 2 Topography of Cerebral Cortex Telencephalon Lateral View Medial View 3 Locating Yourself in the Brain 4 Folds in Cerebral Cortex •Gyrus (Gyri) •Inferior Frontal •Superior Temporal •Sulcus (Sulci) •Central Sulcus •Parieto-Occipital •Pre-Occipital Notch 5 Cytoarchitectonics of the Cerebral Cortex Brodmann (1909) 6 Primary Motor and Somatosensory Areas •Primary Motor Cortex •Precentral Gyrus •Brodmann Area 4 •Premotor Cortex •Brodmann Area 6 •Brodmann Area 8 •Postcentral Gyrus •Brodmann Areas 1-3 7 Consequences of Stroke Insufficient Blood Supply to Brain • Paralysis – voluntary motor function • Anesthesia – tactile sensation • Aphasia – speech, language 8 Sensory and Motor Homunculus Penfield & Jasper (1954) Parietal Frontal Lobe Lobe Longitudinal Fissure Central Fissure Lateral Fissure Scientific American 11/79 9 Primary Auditory and Visual Areas •Primary Auditory Cortex •Superior Temporal Gyrus •Heschl’s Gyrus (AI) •Brodmann Areas 41, 42 •“Tonotopic” Organization •Primary Visual Cortex •Striate Cortex (VI) •Brodmann Area 17 •“Retinotopic” Organization •Extrastriate Cortex •Brodmann’s Areas 18, 19 10 Specialization in Extra-Striate Cortex 11 “Association Areas” -
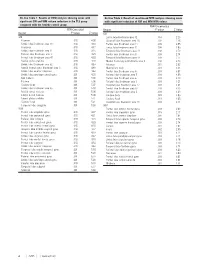
On-Line Table 1: Results of VBM Analysis Showing Areas With
On-line Table 1: Results of VBM analysis showing areas with On-line Table 2: Result of voxel-based MTR analysis showing areas significant GM and WM volume reduction in the TLE group with significant reduction of GM and WM MTR values compared with the healthy control group FDR-Uncorrected FDR-Corrected Region P Value Z Value Region P Value Z Value GM GM Limbic lobe Brodmann area 32 .014 2.20 Putamen .018 4.08 Occipital lobe Brodmann area 18 .007 2.48 Limbic lobe Brodmann area 24 .018 4.16 Frontal lobe Brodmann area 11 .005 2.55 Thalamus .018 4.17 Limbic lobe Brodmann area 31 .004 2.65 Limbic lobe Brodmann area 31 .018 4.25 Temporal lobe Brodmann area 41 .003 2.70 Parietal lobe Brodmann area 3 .018 4.25 Frontal lobe Brodmann area 8 .003 2.74 Frontal lobe Brodmann area 47 .018 4.28 Temporal lobe Brodmann area 34 .003 2.77 Medial globus pallidus .018 4.28 Medial frontal gyrus Brodmann area 9 .003 2.79 Limbic lobe Brodmann area 32 .018 4.63 Putamen .001 2.92 Medial frontal gyrus Brodmann area 9 .018 4.63 Mammillary body .001 3.04 Limbic lobe anterior cingulate .018 4.63 Frontal lobe Brodmann area 4 .001 3.07 Limbic lobe parahippocampal gyrus .001 4.95 Parietal lobe Brodmann area 5 .001 3.09 Red nucleus .001 5.00 Frontal lobe Brodmann area 6 .001 3.20 Pulvinar .001 5.06 Parietal lobe Brodmann area 3 .001 3.27 Caudate body .001 5.07 Occipital lobe Brodmann area 17 .001 3.41 Limbic lobe Brodmann area 35 .001 5.08 Frontal lobe Brodmann area 47 .001 3.49 Medial dorsal nucleus .001 5.08 Parietal lobe Brodmann area 2 .001 3.49 Lateral dorsal nucleus -

Contrasting Patterns of Cortical Input to Architectural Subdivisions of the Area 8 Complex: a Retrograde Tracing Study in Marmoset Monkeys
Cerebral Cortex August 2013;23:1901–1922 doi:10.1093/cercor/bhs177 Advance Access publication June 26, 2012 Contrasting Patterns of Cortical Input to Architectural Subdivisions of the Area 8 Complex: A Retrograde Tracing Study in Marmoset Monkeys David H. Reser, Kathleen J. Burman, Hsin-Hao Yu, Tristan A. Chaplin, Karyn E. Richardson, Katrina H. Worthy and Marcello G.P. Rosa Department of Physiology, Monash University, Clayton, Victoria 3800, Australia Address correspondence to David H. Reser. Email: [email protected] Contemporary studies recognize 3 distinct cytoarchitectural and Yeterian 1996; Petrides and Pandya 1999; Burman et al. 2006; functional areas within the Brodmann area 8 complex, in the Burman and Rosa 2009a). Reflecting these earlier findings, caudal prefrontal cortex: 8b, 8aD, and 8aV. Here, we report on the and the conclusions of the present study, we refer to these quantitative characteristics of the cortical projections to these subdivisions as areas, and regard BA8 as a complex. areas, using injections of fluorescent tracers in marmoset monkeys. The current nomenclature for the areas forming BA8 is cen- Area 8b was distinct from both 8aD and 8aV due to its connections tered on a prime distinction between lateral and medial por- with medial prefrontal, anterior cingulate, superior temporal poly- tions of this complex (designated areas 8A and 8B by Walker sensory, and ventral midline/retrosplenial areas. In contrast, areas 1940), and further evidence that Walker’s area 8A contains Downloaded from 8aD and 8aV received the bulk of the projections from posterior anatomically and functionally distinct dorsal and ventral sub- parietal cortex and dorsal midline areas.