Chemical Analyses of Amelung Glasses: III
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The ISCC-NBS Method of Designating Colors and a Dictionary of Color Names
Uc 8 , .Department of Commerce Na Canal Bureau of Standards Circular UNITED STATES DEPARTMENT OF COMMERCE • Sinclair Weeks, Secretary NATIONAL BUREAU OF STANDARDS • A. V. Astin, Director The ISCC-NBS Method of Designating Colors and a Dictionary of Color Names National Bureau of Standards Circular 553 Issued November 1, 1955 For sale by the Superintendent of Documents, U. S. Government Printing Office, Washington 25, D. C. Price 32 7 1 National Bureau of Standards NOV 1 1955 8 (0*118 QC 00 U555 Cop. 1 Preface I^Ever since the language of man began to develop, words or expressions have been used first to indicate and then to describe colors. Some of these have per- sisted throughout the centuries and are those which refer to the simple colors or ranges such as red or yellow. As the language developed, more and more color names were invented to describe the colors used by art and industry and in late years in the rapidly expanding field of sales promotion. Some of these refer to the pigment or dye used, as Ochre Red or Cochineal, or a geographical location of its source such as Naples Yellow or Byzantium. Later when it became clear that most colors are bought by or for women, many color names indicative of the beauties and wiles of the fan- sex were introduced, as French Nude, Heart’s Desire, Intimate Mood, or Vamp. Fanciful color names came into vogue such as Dream Fluff, Happy Day, Pearly Gates, and Wafted Feather. Do not suppose that these names are without economic importance for a dark reddish gray hat for Milady might be a best seller ; if advertised as Mauve Wine whereas it probably would not if the color were called Paris Mud. -

Made in America
Made in America www.bobbincentral.com Color SKU 1000m 5000m White 8 10000 Super White 10002 German Granite 8 10401 Medium Grey 8 10424 Battleship 10430 Titanium 8 10431 Flint 10435 Silver 10536 Mercury 10643 Sterling 10877 Cool Grey 3 8 10CG3 Fog 10CG6 Cool Grey 7 8 10CG7 Cool Grey 9 10CG9 Linen 8 10WG1 Warm Grey 4 8 10WG4 Warm Grey 6 10WG6 Black 8 11001 Slate 15285 Anchor 15295 Nickel 15497 Bone 17443 Light Grey 8 17543 Shadow 1BLK3 Storm 1BLK7 Lead Grey 8 1CG11 Warm Grey 11 1WG11 Cream 8 20001 Pearl 20005 Leather 8 20140 Mahogany 8 20160 Medium Brown 8 20464 Sand 8 20466 Caramel 20467 Biscotti 20468 Chocolate 20469 Hazel 20470 Apricot Blush 20474 Dark Brown 8 20476 Rust Brown 8 20478 MoCha 8 20727 Light Copper 20730 Vegas Gold 20872 Dijon 21245 Antique 21255 Sienna 21615 Sepia 21685 Cleopatra 8 24515 Khaki 8 24525 Shell 24535 Brunette 8 24625 Bark 24635 Light Tan 8 24655 Camel 24665 Cork 24675 Cocoa 24705 Military Gold 8 27407 Wheat 27500 Sand Dune 27501 Coffee 27504 Butterscotch 27508 Coffee Bean 27518 Chestnut 8 27521 Auburn 27523 Brownie 27596 Latte 29181 Rock Navy 30001 Blueberry 8 30281 Azure 30283 Hawaiian Blue 8 30284 Empire 30286 Bombay 30287 Bright Blue 8 30288 Baby Blue 30290 Midnight Navy 30296 Cerulean 30308 Magic Mint 30317 Eclipse 30532 Denim 30534 Robin Egg 8 30632 Graphite 30644 Sky 30646 Cobalt 30647 Admiral 30654 Captain Navy 30655 Royal 30661 Lagoon 32237 Air ForCe Blue 32382 Federal 32757 Deep Sea 32767 Navy 8 32965 Light Turquoise 8 32975 Electric 33015 Zaffre 35405 Cloud 8 37457 Ocean 37468 Aquamarine 37474 Steel -

European Cobalt Sources Identified in the Production of Chinese Famille Rose Porcelain Abstract Keywords
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by UCL Discovery 1 European cobalt sources identified in the production of 2 Chinese famille rose porcelain 3 4 Rita Giannini1, Ian C. Freestone2, Andrew J. Shortland1 5 6 1 Cranfield Forensic Institute 7 Cranfield University 8 Defence Academy of the United Kingdom 9 Shrivenham 10 Wilts 11 SN6 8LA 12 [email protected] 13 14 2 Institute of Archaeology 15 UCL 16 31-34 Gordon Square 17 London WC1H 0PY 18 Abstract 19 The blue pigments on 112 fragments or small objects of Qing Dynasty Chinese, 20 95 of underglaze blue and white and 17 overglaze enamelled porcelains were 21 analysed by LA-ICPMS. The underglaze blues on both blue and white and 22 polychrome objects were created with a cobalt pigment that was rich in 23 manganese with lesser nickel and zinc. This suite of accessory elements is 24 generally considered to be characteristic of local, Chinese, sources of pigments. 25 However, the blue enamels were very different. The cobalt pigment here has low 26 levels of manganese and instead is rich in nickel, zinc, arsenic and bismuth. No 27 Chinese source of cobalt with these characteristics is known, but they closely 28 match the elements found in the contemporary cobalt source at Erzgebirge in 29 Germany. Textual evidence has been interpreted to suggest that some enamel 30 pigment technologies were transferred from Europe to China, but this is the first 31 analytical evidence to be found that an enamel pigment itself was imported. -
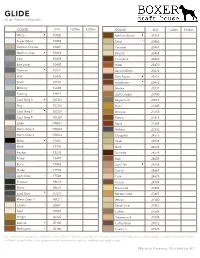
Thread Color Chart BCH.Pdf
GLIDE 40 wt. Filament Polyester Order a color card for a true color match Magna-Glide Delights available in this color COLOR SKU 1,000m 5,000m COLOR SKU 1,000m 5,000m White 10000 Medium Brown 20464 Super White 10002 Sand 20466 German Granite 10401 Caramel 20467 Medium Grey 10424 Biscotti 20468 Coin 10429 Chocolate 20469 Battleship 10430 Hazel 20470 Titanium 10431 Apricot Blush 20474 Flint 10435 Dark Brown 20476 Silver 10536 Rust Brown 20478 Mercury 10643 Mocha 20727 Sterling 10877 Light Copper 20730 Cool Grey 3 10CG3 Vegas Gold 20872 Fog 10CG6 Dijon 21245 Cool Grey 7 10CG7 Antique 21255 Cool Grey 9 10CG9 Sienna 21615 Linen 10WG1 Sepia 21685 Warm Grey 4 10WG4 Hickory 22336 Warm Grey 6 10WG6 Cleopatra 24515 Black 11001 Khaki 24525 Slate 15285 Shell 24535 Anchor 15295 Brunette 24625 Nickel 15497 Bark 24635 Bone 17443 Light Tan 24655 Husky 17530 Camel 24665 Light Grey 17543 Cork 24675 Shadow 1BLK3 Cocoa 24705 Storm 1BLK7 Buttermilk 27403 Lead Grey 1CG11 Military Gold 27407 Warm Grey 11 1WG11 Wheat 27500 Cream 20001 Sand Dune 27501 Pearl 20005 Coffee 27504 Ginger 20125 Butterscotch 27508 Leather 20140 Coffee Bean 27518 Mahogany 20160 Chestnut 27521 Our color chart is designed to help you reference Glide™ thread colors. We offer this chart as a reference only, and it is not intended to imply an exact color match of Glide™ thread colors. Color appearance will vary based on lighting conditions and angle of view. www.habanddash.com Made in America, Stitched by All GLIDE 40 wt. Filament Polyester Order a color card for a true color match Magna-Glide -
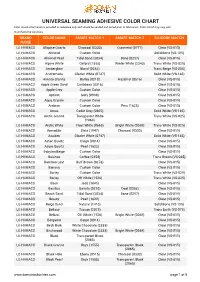
UNIVERSAL SEAMING ADHESIVE COLOR CHART Color Match Information Is Provided As Reference Only and Should Be Verifed and Tested Prior to Fabrication
LG HI- MACS UNIVERSAL SEAMING ADHESIVE COLOR CHART Color match information is provided as reference only and should be verifed and tested prior to fabrication. Color match may vary with manufacturing variations BRAND COLOR NAME SMART MATCH 1 SMART MATCH 2 SILICONE MATCH NAME LG HI-MACS Allspice Quartz Charcoal (0333) Gunmetal (0777) Clear (VS-015) LG HI-MACS Almond Custom Color Solid Bone (VS-185) LG HI-MACS Almond Pearl Tidal Sand (2034) Bone (0257) Clear (VS-015) LG HI-MACS Alpine White Oxford (1588) Winter White (2340) Trans White (VS-025) LG HI-MACS Amberglow Blond (0248) Trans Beige (VS-085) LG HI-MACS Andromeda Glacier White (0747) Solid White (VS-165) LG HI-MACS Annato Granite Barley (0218) Hazelnut (0816) Clear (VS-015) LG HI-MACS Apple Green Sand Caribbean (0316) Clear (VS-015) LG HI-MACS Apple Grey Custom Color Clear (VS-015) LG HI-MACS Apricot Ivory (0980) Clear (VS-015) LG HI-MACS Aqua Granite Custom Color Clear (VS-015) LG HI-MACS Arabian Custom Color Peru (1625) Clear (VS-015) LG HI-MACS Arcas Cadet Grey (0305) Solid White (VS-165) LG HI-MACS Arctic Granite Transparent White Trans White (VS-025) (2068) LG HI-MACS Arctic White Frost White (0665) Bright White (0268) Trans White (VS-025) LG HI-MACS Armadillo Slate (1947) Charcoal (0333) Clear (VS-015) LG HI-MACS Awaken Glacier White (0747) Solid White (VS-165) LG HI-MACS Aztec Quartz Cargo (0314) Clear (VS-015) LG HI-MACS Azure Quartz Pearl (1622) Clear (VS-015) LG HI-MACS Babylon Beige Custom Color Clear (VS-015) LG HI-MACS Balance Coffee (0354) Trans Brown (VS-065) LG HI-MACS -

Air Force Blue (Raf) {\Color{Airforceblueraf}\#5D8aa8
Air Force Blue (Raf) {\color{airforceblueraf}\#5d8aa8} #5d8aa8 Air Force Blue (Usaf) {\color{airforceblueusaf}\#00308f} #00308f Air Superiority Blue {\color{airsuperiorityblue}\#72a0c1} #72a0c1 Alabama Crimson {\color{alabamacrimson}\#a32638} #a32638 Alice Blue {\color{aliceblue}\#f0f8ff} #f0f8ff Alizarin Crimson {\color{alizarincrimson}\#e32636} #e32636 Alloy Orange {\color{alloyorange}\#c46210} #c46210 Almond {\color{almond}\#efdecd} #efdecd Amaranth {\color{amaranth}\#e52b50} #e52b50 Amber {\color{amber}\#ffbf00} #ffbf00 Amber (Sae/Ece) {\color{ambersaeece}\#ff7e00} #ff7e00 American Rose {\color{americanrose}\#ff033e} #ff033e Amethyst {\color{amethyst}\#9966cc} #9966cc Android Green {\color{androidgreen}\#a4c639} #a4c639 Anti-Flash White {\color{antiflashwhite}\#f2f3f4} #f2f3f4 Antique Brass {\color{antiquebrass}\#cd9575} #cd9575 Antique Fuchsia {\color{antiquefuchsia}\#915c83} #915c83 Antique Ruby {\color{antiqueruby}\#841b2d} #841b2d Antique White {\color{antiquewhite}\#faebd7} #faebd7 Ao (English) {\color{aoenglish}\#008000} #008000 Apple Green {\color{applegreen}\#8db600} #8db600 Apricot {\color{apricot}\#fbceb1} #fbceb1 Aqua {\color{aqua}\#00ffff} #00ffff Aquamarine {\color{aquamarine}\#7fffd4} #7fffd4 Army Green {\color{armygreen}\#4b5320} #4b5320 Arsenic {\color{arsenic}\#3b444b} #3b444b Arylide Yellow {\color{arylideyellow}\#e9d66b} #e9d66b Ash Grey {\color{ashgrey}\#b2beb5} #b2beb5 Asparagus {\color{asparagus}\#87a96b} #87a96b Atomic Tangerine {\color{atomictangerine}\#ff9966} #ff9966 Auburn {\color{auburn}\#a52a2a} #a52a2a Aureolin -

Swatch Name HLS RGB HEX Absolute Zero 217° 36% 100% 0 72
Swatch Name HLS RGB HEX Absolute Zero 217° 36% 100% 0 72 186 #0048BA Acid green 65° 43% 76% 176 191 26 #B0BF1A Aero 206° 70% 70% 124 185 232 #7CB9E8 Aero blue 151° 89% 100% 201 255 229 #C9FFE5 African violet 288° 63% 31% 178 132 190 #B284BE Air superiority blue 205° 60% 39% 114 160 193 #72A0C1 Alabaster 46° 90% 27% 237 234 224 #EDEAE0 Alice blue 208° 97% 100% 240 248 255 #F0F8FF Alloy orange 27° 42% 85% 196 98 16 #C46210 Almond 30° 87% 52% 239 222 205 #EFDECD Amaranth 348° 53% 78% 229 43 80 #E52B50 Amaranth (M&P) 328° 40% 57% 159 43 104 #9F2B68 Amaranth pink 338° 78% 75% 241 156 187 #F19CBB Amaranth purple 342° 41% 63% 171 39 79 #AB274F Amaranth red 356° 48% 73% 211 33 45 #D3212D Amazon 147° 35% 35% 59 122 87 #3B7A57 Amber 45° 50% 100% 255 191 0 #FFBF00 Amber (SAE/ECE) 30° 50% 100% 255 126 0 #FF7E00 Amethyst 270° 60% 50% 153 102 204 #9966CC Android green 74° 50% 55% 164 198 57 #A4C639 Antique brass 22° 63% 47% 205 149 117 #CD9575 Antique bronze 52° 26% 55% 102 93 30 #665D1E Antique fuchsia 316° 46% 22% 145 92 131 #915C83 Antique ruby 350° 31% 66% 132 27 45 #841B2D Antique white 34° 91% 78% 250 235 215 #FAEBD7 Ao (English) 120° 25% 100% 0 128 0 #008000 Apple green 74° 36% 100% 141 182 0 #8DB600 Apricot 24° 84% 90% 251 206 177 #FBCEB1 Aqua 180° 50% 100% 0 255 255 #00FFFF Aquamarine 160° 75% 100% 127 255 212 #7FFFD4 Swatch Name HLS RGB HEX Arctic lime 72° 54% 100% 208 255 20 #D0FF14 Army green 69° 23% 44% 75 83 32 #4B5320 Artichoke 76° 53% 13% 143 151 121 #8F9779 Arylide yellow 51° 67% 74% 233 214 107 #E9D66B Ash gray 135° 72% 8% 178 190 -

(12) Patent Application Publication (10) Pub. No.: US 2014/0350127 A1 CANO Et Al
US 2014035O127A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2014/0350127 A1 CANO et al. (43) Pub. Date: Nov. 27, 2014 (54) COLORANT COMPOUNDS DERVED FROM Publication Classification GENPIN OR GENPIN CONTAINING MATERLALS (51) Int. Cl. C09B 23/04 (2006.01) (71) Applicant: ECOFLORA S.A.S., Sabaneta (CO) C09B 67/54 (2006.01) C09B 67/22 (2006.01) (72) Inventors: Esteban Vargas CANO, Itagui (CO); (52) U.S. Cl. Luis Fernando Echeverri Lopez, CPC ............. C09B 23/04 (2013.01); C09B 67/0034 Medellin (CO); Juan Fernando Gil (2013.01); C09B 67/0096 (2013.01) Romero, Medellin (CO); Edwin Andrés USPC ........ 514/772.7:528/327: 528/321:524/879; Correa Garcés, Medellin (CO); Sandra 435/267; 426/540 Patricia Zapata Porras, Medellin (CO) (57) ABSTRACT (73) Assignee: ECOFLORA S.A.S., Sabaneta (CO) The present disclosure provides colorant compounds and methods of isolation of the colorant compounds derived from (21) Appl. No.: 14/285,325 a reaction of genipin and an amine. The colorant composi tions comprise purified compounds (e.g., a purified polymer (22) Filed: May 22, 2014 or a purified dimer) obtained from multiple fractioning by chromatography of the reaction resulting material. The puri Related U.S. Application Data fied polymer or dimer can be used as a colorant by itself or in (60) Provisional application No. 61/826.391, filed on May combination with another colorant for imparting color to a 22, 2013, provisional application No. 61/836,072, food, a drug, a cosmetic, a medical device, and textile prod filed on Jun. -

The Named Colors List Contains Over 1.500 Color Names with HEX and RGB Values, from "Absolute Zero" to "Zomp"
The Named Colors list contains over 1.500 color names with HEX and RGB values, from "Absolute Zero" to "Zomp". Color Preview Color Name Hex RGB Absolute Zero 0048BA 0, 72, 186 Acid green B0BF1A 176, 191, 26 Aero 7CB9E8 124, 185, 232 Aero blue C9FFE5 201, 255, 229 African violet B284BE 178, 132, 190 Air superiority blue 72A0C1 114, 160, 193 Alabama crimson AF002A 175, 0, 42 Alabaster F2F0E6 242, 240, 230 Aliceblue F0F8FF 240, 248, 255 Alloy orange C46210 196, 98, 16 Almond EFDECD 239, 222, 205 Aloeswood brown (Tonocha) 5A6457 90, 100, 87 Aloewood-color (Kyara-iro) 6A432D 106, 67, 45 Amaranth E52B50 229, 43, 80 Amaranth deep purple 9F2B68 159, 43, 104 Amaranth pink F19CBB 241, 156, 187 Amaranth purple AB274F 171, 39, 79 Amaranth red D3212D 211, 33, 45 Amazon 3B7A57 59, 122, 87 Amber FFBF00 255, 191, 0 Amber (Kohaku-iro) CA6924 202, 105, 36 Amber (SAE/ECE) FF7E00 255, 126, 0 Amethyst 9966CC 153, 102, 204 Amur cork tree (Kihada) F3C13A 243, 193, 58 Anti-flash white F2F3F4 242, 243, 244 Antique brass CD9575 205, 149, 117 Antique bronze 665D1E 102, 93, 30 Antique fuchsia 915C83 145, 92, 131 Color Preview ACnotlioqru eN arumbey H8e4x1B2D R1G32B, 27, 45 Antiquewhite FAEBD7 250, 235, 215 Apple 66B447 102, 180, 71 Apple green 8DB600 141, 182, 0 Apricot FBCEB1 251, 206, 177 Aqua 00FFFF 0, 255, 255 Aqua Blue color (Mizu-iro) 86ABA5 134, 171, 165 Aquamarine 7FFFD4 127, 255, 212 Arctic lime D0FF14 208, 255, 20 Army green 4B5320 75, 83, 32 Artichoke 8F9779 143, 151, 121 Arylide yellow E9D66B 233, 214, 107 Ash gray B2BEB5 178, 190, 181 Asparagus 87A96B -

Fabric Collections
BEACH COLLECTION FEATURES / CARACTERÍSTICAS VALUE / VALOR STANDARD / NORMA Composition 100% Solution Dyed Acrylic Composición 100% Acrílica Tintada en Masa Weight 250 g/m2 UNE-EN ISO 40339 Peso Available widths Anchos disponibles 55” / 140 cm Roll length 50 m Longitud de pieza Resistance to surface wetting (Spray Test) 100 UNE-EN ISO 4920 Resistencia al mojado superficial Oil repellency >_ Resistencia al aceite 5 AATCC 118 Colour fastness to artificial weathering 7-8 blue scale (8) UNE-EN ISO 105-B04 Premiere Solidez del color a la intemperie artificial Colour fastness to light 7-8 blue scale (8) UNE-EN ISO 105-B02 Solidez del color a la luz Flame retardant Class 1 NFPA 260 Comportamiento al fuego Pass EN 1021-1 Pass BS 5852-4 Prints Pass CA TB 117 Tolerance +/- 5% Average Values. No contract information. Tolerancia de +/- 5% en todos los valores. Valores medios. Información no contractual. The data are the result of tests performed in our laboratories. Los datos obtenidos son el resultado de los ensayos realizados en nuestros laboratorios. For more technical information, please contact us at [email protected] . Para más información técnica, por favor, contacte directamente con nosotros a través del correo [email protected] . Quality - Calidad Warranty The warranty exclusively covers the abnormal color and fabric resistance loss, given a normal exposure to the action of sun, salinity and general atmospheric agents. Outdoor The warranty exclusively covers the Docril® G fabric. Docril® G may be a component of an end product such as indoor and outdoor furniture. The warranty must be claimed against the retailer or installer, whom the end product is purchased from. -
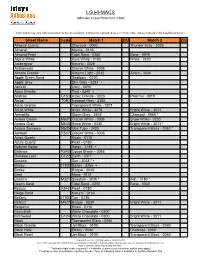
LG HI-MACS Adhesive Cross Reference Chart
LG HI-MACS Adhesive Cross Reference Chart Color match may vary with manufacturers dye lot variations. Information is provided as a reference only; end user should verify suitability prior use. Sheet Name Code Match 1 Z/U Match 2 Z/U Allspice Quartz Charcoal - 0060 Thunder Grey - 3036 Almond Manila - 0133 Almond Pearl Tidal Sand - 0250 Bone - 0040 Alpine White Pure White - 0190 White - 2420 Amberglow Neutral - 3029 Andromeda Glacier White - 3006 Annato Granite Autumn Light - 2032 Acorn - 3034 Apple Green Sand Seafoam - 0220 Apple Grey Dim Grey - 3283 + Apricot Ivory - 0090 Aqua Granite Teal - 0240 + Arabian G153 Aztec Granite - 3028 Palermo - 3019 Arcas T050 Newport Grey - 2360 Arctic Granite Transparent White - 0271 Arctic White Arctic White - 3076 Bright White - 3011 Armadillo Storm Grey - 2402 Charcoal - 0060 * Aurora Cream M607 Glacier White - 3006 Snow White - 0230 Aurora Gray M608 Snow White - 0230 Bright White - 3011 * Aurora Sanremo M605 Uba Tuba - 2405 Transparent Black - 0260 * Awaken R537 Glacier White - 3006 Aztec Quartz Khaki - 0110 Azure Quartz Pearl - 0150 Babylon Beige Safari - 3189 + Balance R540 Cocoa Brown - 0065 Bamboo Leaf G122 Earth - 3077 Banana Sun - 3038 * + Barley GT937 Barley - 3265 + Barley Bisque - 0030 Basil Moss - 0131 Basilica M321 Seashell - 3035 * Ash - 3180 * Beach Sand Tidal Sand - 0250 Bone - 0040 Beauty R543 Pearl - 0150 Beige Sand Natural - 0130 Bellany GT933 Tan - 0235 Bellizzi M427 Bisque - 0030 Bright White - 3011 Bergamo Khaki - 0110 Birch Bark White Chocolate - 0300 Birchwood G124 White Chocolate - 0300 Bright White - 3011 Black Transparent Black - 0260 Black Granite Jet Black - 0100 Transparent Black - 0260 Black Hole Ebony - 0080 Charcoal - 0060 Black Pearl Jet Black - 0100 Transparent Black - 0260 Integra Adhesives Inc. -

Cobalt and Associated Impurities in Blue (And
Cobalt and Associated Impurities in Blue (and Green) Glass, Glaze and Enamel: Relationships between Raw Materials, Processing, Composition, Phases and International Trade Philippe Colomban, Burcu Kırmızı, Gulsu Simsek Franci To cite this version: Philippe Colomban, Burcu Kırmızı, Gulsu Simsek Franci. Cobalt and Associated Impurities in Blue (and Green) Glass, Glaze and Enamel: Relationships between Raw Materials, Processing, Composi- tion, Phases and International Trade. Minerals, MDPI, 2021, 11 (6), pp.633. 10.3390/min11060633. hal-03285991 HAL Id: hal-03285991 https://hal.archives-ouvertes.fr/hal-03285991 Submitted on 13 Jul 2021 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Review Cobalt and Associated Impurities in Blue (and Green) Glass, Glaze and Enamel: Relationships between Raw Materials, Processing, Composition, Phases and International Trade Philippe Colomban 1,*, Burcu Kırmızı 2 and Gulsu Simsek Franci 1,3 1 MONARIS UMR8233, Sorbonne Université, CNRS, 4 Place Jussieu, 75005 Paris, France 2 Department of Conservation and Restoration of