(10) Patent No.: US 9376569 B2
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

To Our People-Animal Connection
Bi-Annual Newsletter · July 2017 TO OUR PEOPLE-ANIMAL CONNECTION FAMILY The UCLA People-Animal Connection (PAC) is dedicated to providing compassionate, world-class patient care, companionship, and warmth to more than 1,000 critically ill children and adults every month and is grateful for your support. PAC ATTENDS UCLA STUDENT-ATHLETE HEALTH AND WELLNESS FAIR FOR FIRST TIME Members from the UCLA Softball team pose with PAC dogs Shepzel and Mazel. In March, the People-Animal Connection (PAC) teams had the privilege of attending the UCLA Student-Athlete Health and Wellness Fair at the J.D. Morgan Center, which serves as the “hub” for all 25 of UCLA’s intercollegiate athletic teams. The event enabled students to engage in an open conversation about mental health and substance abuse. Attending the event for the first time, PAC teams brought comfort and cheer to student-athletes heading into the winter quarter finals week. As the dogs ran around, interacting with the athletes, everyone enjoyed opportunities for plentiful petting, great conversations, and delightful Snapchat stories. Many dogs sported UCLA blue and gold accessories or wore UCLA-inspired attire, showing their support for the athletes and rooting for their success. The program is grateful for the chance to bring the soothing and friendly K-9 companionship to student-athletes. Thanks to all the wonderful PAC volunteers who contributed to the success of this event! They are hoping to return for another installment next year. PICK PICO! On May 21, PAC teams participated for the third year in the Pick Pico Event, a community celebration that features great local food and products, live entertainment, and a chance to learn about local government and nonprofit organizations. -

BRAND GUIDELINES Version 21.0 08/06/15 TABLE of CONTENTS
The CENTENNIAL Campaign for BRAND GUIDELINES Version 21.0 08/06/15 TABLE OF CONTENTS BRAND BASICS 3 COPY TONE 55 Copy Tone 55 CAMPAIGN AT A GLANCE 4 Writing Compelling Headlines 56 Body Copy and Long Form 57 CENTENNIAL CAMPAIGN POSITIONING 5 TEMPLATES 58 CONCEPT AND OVERARCHING THEME 6 Email Signatures 58 Donor Proposal 59 Stationery 60 CREATIVE EXECUTION 7 Unit Case Statement 62 Email Templates 63 FACTS AND FIGURES 8 CAMPAIGN BACKGROUND AND PLAN 9 EVERGREEN CONTENT 10 LOGOS & LOCKUPS 12 Let There Be Logotype 12 Let There Be Logotype with Ellipsis 13 The centennial campaign lockup 20 TYPOGRAPHY 23 Brand Typography 23 Campaign Typography 24 Alternatives 25 Headlines Applications 27 Body Copy 32 Hierarchy 32 Digital Typography 33 COLORS 36 Color Usage 36 GRAPHIC ELEMENTS 39 Photons 40 Molecules 42 Slanted Overlay Box 44 Facets 46 IMAGERY 48 Campus and Los Angeles 48 People 49 Sky/Sun 51 Usage / Image Cropping 53 2 The Centennial Campaign for UCLA Brand Guidelines BRAND BASICS WHAT IS A BRAND? UCLA views the world differently. Where most see the world how it is, we see it as it could be. Some may call this optimistic. We call it essential. And it is what has enabled us to effect change time and time again. Whether it’s being the birthplace of the Internet or the home to more NCAA titles than any other university; licensing over 500 active patents or saving countless lives a year—everything we do here comes from unflappable belief that if we set our sights on something, it will be done. -

The ISCC-NBS Method of Designating Colors and a Dictionary of Color Names
Uc 8 , .Department of Commerce Na Canal Bureau of Standards Circular UNITED STATES DEPARTMENT OF COMMERCE • Sinclair Weeks, Secretary NATIONAL BUREAU OF STANDARDS • A. V. Astin, Director The ISCC-NBS Method of Designating Colors and a Dictionary of Color Names National Bureau of Standards Circular 553 Issued November 1, 1955 For sale by the Superintendent of Documents, U. S. Government Printing Office, Washington 25, D. C. Price 32 7 1 National Bureau of Standards NOV 1 1955 8 (0*118 QC 00 U555 Cop. 1 Preface I^Ever since the language of man began to develop, words or expressions have been used first to indicate and then to describe colors. Some of these have per- sisted throughout the centuries and are those which refer to the simple colors or ranges such as red or yellow. As the language developed, more and more color names were invented to describe the colors used by art and industry and in late years in the rapidly expanding field of sales promotion. Some of these refer to the pigment or dye used, as Ochre Red or Cochineal, or a geographical location of its source such as Naples Yellow or Byzantium. Later when it became clear that most colors are bought by or for women, many color names indicative of the beauties and wiles of the fan- sex were introduced, as French Nude, Heart’s Desire, Intimate Mood, or Vamp. Fanciful color names came into vogue such as Dream Fluff, Happy Day, Pearly Gates, and Wafted Feather. Do not suppose that these names are without economic importance for a dark reddish gray hat for Milady might be a best seller ; if advertised as Mauve Wine whereas it probably would not if the color were called Paris Mud. -

Made in America
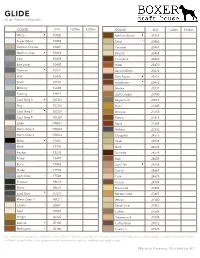
Made in America www.bobbincentral.com Color SKU 1000m 5000m White 8 10000 Super White 10002 German Granite 8 10401 Medium Grey 8 10424 Battleship 10430 Titanium 8 10431 Flint 10435 Silver 10536 Mercury 10643 Sterling 10877 Cool Grey 3 8 10CG3 Fog 10CG6 Cool Grey 7 8 10CG7 Cool Grey 9 10CG9 Linen 8 10WG1 Warm Grey 4 8 10WG4 Warm Grey 6 10WG6 Black 8 11001 Slate 15285 Anchor 15295 Nickel 15497 Bone 17443 Light Grey 8 17543 Shadow 1BLK3 Storm 1BLK7 Lead Grey 8 1CG11 Warm Grey 11 1WG11 Cream 8 20001 Pearl 20005 Leather 8 20140 Mahogany 8 20160 Medium Brown 8 20464 Sand 8 20466 Caramel 20467 Biscotti 20468 Chocolate 20469 Hazel 20470 Apricot Blush 20474 Dark Brown 8 20476 Rust Brown 8 20478 MoCha 8 20727 Light Copper 20730 Vegas Gold 20872 Dijon 21245 Antique 21255 Sienna 21615 Sepia 21685 Cleopatra 8 24515 Khaki 8 24525 Shell 24535 Brunette 8 24625 Bark 24635 Light Tan 8 24655 Camel 24665 Cork 24675 Cocoa 24705 Military Gold 8 27407 Wheat 27500 Sand Dune 27501 Coffee 27504 Butterscotch 27508 Coffee Bean 27518 Chestnut 8 27521 Auburn 27523 Brownie 27596 Latte 29181 Rock Navy 30001 Blueberry 8 30281 Azure 30283 Hawaiian Blue 8 30284 Empire 30286 Bombay 30287 Bright Blue 8 30288 Baby Blue 30290 Midnight Navy 30296 Cerulean 30308 Magic Mint 30317 Eclipse 30532 Denim 30534 Robin Egg 8 30632 Graphite 30644 Sky 30646 Cobalt 30647 Admiral 30654 Captain Navy 30655 Royal 30661 Lagoon 32237 Air ForCe Blue 32382 Federal 32757 Deep Sea 32767 Navy 8 32965 Light Turquoise 8 32975 Electric 33015 Zaffre 35405 Cloud 8 37457 Ocean 37468 Aquamarine 37474 Steel -

UCLA QUICK FACTS 2008-09 BRUINS 9 2008-09 Schedule
TABLE OF CONTENTS UCLA QUICK FACTS 2008-09 BRUINS 9 2008-09 Schedule .....................Inside Back Cover Address ............ J.D. Morgan Center, PO Box 24044 Season Outlook .......................................................2 Los Angeles, CA 90024-0044 Alphabetical Roster ................................................4 Athletics Phone ...................................(310) 825-8699 Portrait Roster .........................................................4 Ticket Offi ce.................................. (310) UCLA-WIN THE COACHING STAFF Chancellor ...........................................Dr. Gene Block Director of Athletics ..................Daniel G. Guerrero Head Coach Derek Freeman ................................5 Faculty Athletic Rep. ......................Donald Morrison Director of Operations Daniel Hour .................6 Enrollment .......................................................... 37,000 Undergraduate Assistant Coach Founded ................................................................. 1919 Brandon Christianson ............................6 Colors ....................................................Blue and Gold THE PLAYERS Nickname ............................................................ Bruins Conference.....................................................Pacifi c-10 Player Biographies ...................................................7 Conference Phone .................................925-932-4411 THE 2007-08 SEASON Conference Fax ......................................925-932-4601 National Affi -

European Cobalt Sources Identified in the Production of Chinese Famille Rose Porcelain Abstract Keywords
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by UCL Discovery 1 European cobalt sources identified in the production of 2 Chinese famille rose porcelain 3 4 Rita Giannini1, Ian C. Freestone2, Andrew J. Shortland1 5 6 1 Cranfield Forensic Institute 7 Cranfield University 8 Defence Academy of the United Kingdom 9 Shrivenham 10 Wilts 11 SN6 8LA 12 [email protected] 13 14 2 Institute of Archaeology 15 UCL 16 31-34 Gordon Square 17 London WC1H 0PY 18 Abstract 19 The blue pigments on 112 fragments or small objects of Qing Dynasty Chinese, 20 95 of underglaze blue and white and 17 overglaze enamelled porcelains were 21 analysed by LA-ICPMS. The underglaze blues on both blue and white and 22 polychrome objects were created with a cobalt pigment that was rich in 23 manganese with lesser nickel and zinc. This suite of accessory elements is 24 generally considered to be characteristic of local, Chinese, sources of pigments. 25 However, the blue enamels were very different. The cobalt pigment here has low 26 levels of manganese and instead is rich in nickel, zinc, arsenic and bismuth. No 27 Chinese source of cobalt with these characteristics is known, but they closely 28 match the elements found in the contemporary cobalt source at Erzgebirge in 29 Germany. Textual evidence has been interpreted to suggest that some enamel 30 pigment technologies were transferred from Europe to China, but this is the first 31 analytical evidence to be found that an enamel pigment itself was imported. -
Thread Color Chart BCH.Pdf
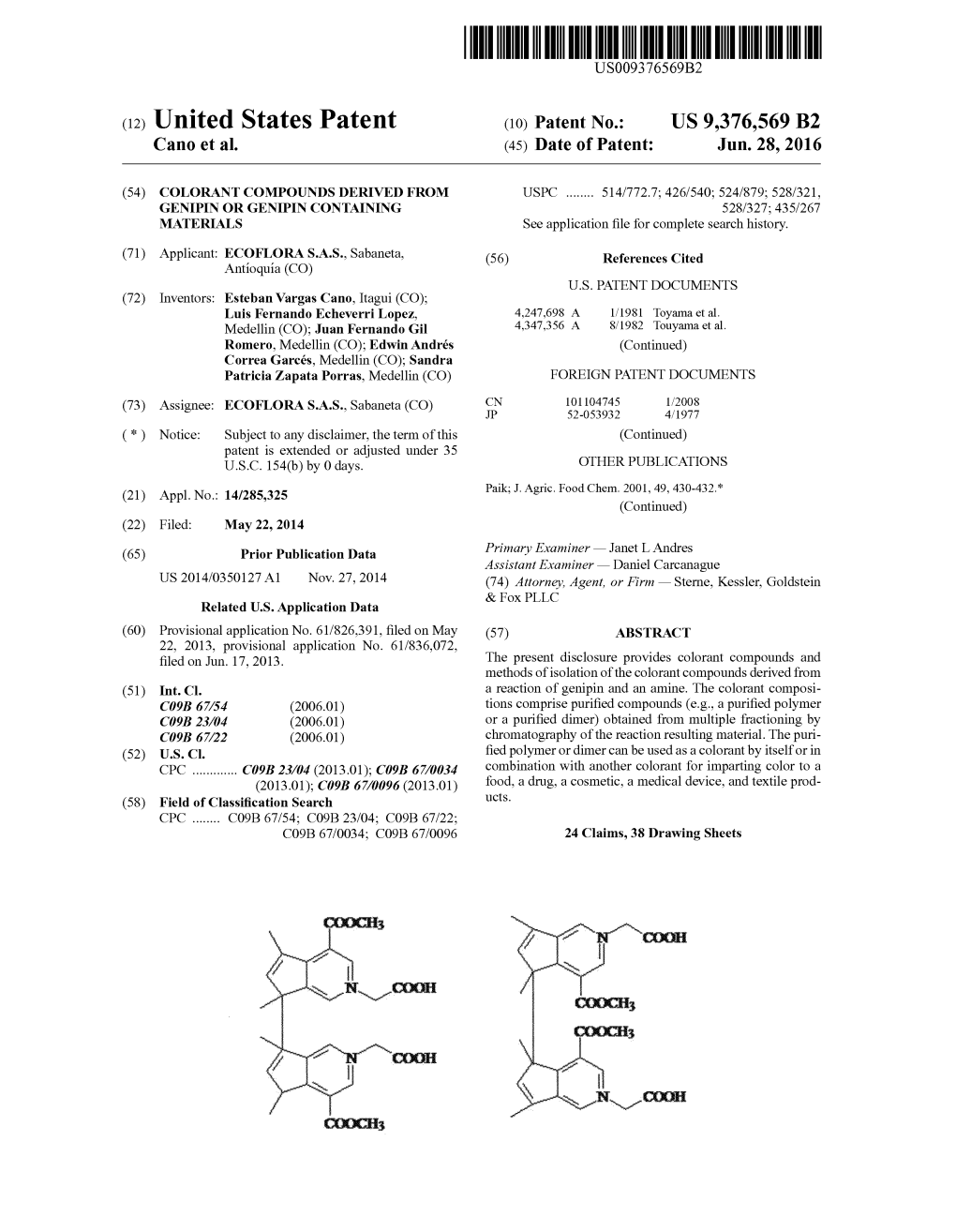
GLIDE 40 wt. Filament Polyester Order a color card for a true color match Magna-Glide Delights available in this color COLOR SKU 1,000m 5,000m COLOR SKU 1,000m 5,000m White 10000 Medium Brown 20464 Super White 10002 Sand 20466 German Granite 10401 Caramel 20467 Medium Grey 10424 Biscotti 20468 Coin 10429 Chocolate 20469 Battleship 10430 Hazel 20470 Titanium 10431 Apricot Blush 20474 Flint 10435 Dark Brown 20476 Silver 10536 Rust Brown 20478 Mercury 10643 Mocha 20727 Sterling 10877 Light Copper 20730 Cool Grey 3 10CG3 Vegas Gold 20872 Fog 10CG6 Dijon 21245 Cool Grey 7 10CG7 Antique 21255 Cool Grey 9 10CG9 Sienna 21615 Linen 10WG1 Sepia 21685 Warm Grey 4 10WG4 Hickory 22336 Warm Grey 6 10WG6 Cleopatra 24515 Black 11001 Khaki 24525 Slate 15285 Shell 24535 Anchor 15295 Brunette 24625 Nickel 15497 Bark 24635 Bone 17443 Light Tan 24655 Husky 17530 Camel 24665 Light Grey 17543 Cork 24675 Shadow 1BLK3 Cocoa 24705 Storm 1BLK7 Buttermilk 27403 Lead Grey 1CG11 Military Gold 27407 Warm Grey 11 1WG11 Wheat 27500 Cream 20001 Sand Dune 27501 Pearl 20005 Coffee 27504 Ginger 20125 Butterscotch 27508 Leather 20140 Coffee Bean 27518 Mahogany 20160 Chestnut 27521 Our color chart is designed to help you reference Glide™ thread colors. We offer this chart as a reference only, and it is not intended to imply an exact color match of Glide™ thread colors. Color appearance will vary based on lighting conditions and angle of view. www.habanddash.com Made in America, Stitched by All GLIDE 40 wt. Filament Polyester Order a color card for a true color match Magna-Glide -

The Desertarian
The Desertarian DECEMBER 3RD, 2008 OFFICERS & SCRIBE: DAVID CUTTER DIRECTORS PHOTOGRAPHER MIKE BRILL 2008– 2009 Jacque Wachs— President Belgian Hugs Greeting us today were Milt of Allegiance. Lea a small smattering of Ray Corvan, President Levinson and Ray Corvan. Berghmans, in between hugs applause. Elect & Program Chair all around, helped us recite Bob Elsner, Immediate the Three Way Test Past President (photogenic pause) of the Jacque introduced our lone Milt Levinson, Secretary Four Things We Think, Say visitor, returning Sojourner, Janie Bolitho, Treasurer, and Do. Club Service Roger Mickalko. It is great to Board of Directors see you again, Roger! Bob Allan Mike Brill Barry Freet Bob Jones introduced his Helene Kalfuss guest, Stephen Lerman, Lars Hansen Sylvia Zarasua special for the final time. President Jacque Wachs Jerry Haugh again introduced Kevin Nay opened our meeting by asking Mark Stanley his wife and chauffeur, Sherry Art Snow to lead the Pledge Haugh, as not special. Ex Officio Jim Dowler Bob Barrett offered a fine DESERTARIAN STAFF Rotary invocation. Mark Stanley Janet Miller David Cutter Ken Sherman The announcement of no Kevin Nay song today was greeted with Devorah Kalani Photographer Mike Brill Our Meeting December 3rd 2008 WRONG! Did you walk Helene Kalfuss brought a showing us the many ways to home, Jerry? special guest, Amanda Barrett makeup Rotary meetings. from California Patios and Devorah Kalani introduced Francis Flynn. It was great to have Bud Wein join us, courtesy of Jim Dowler. Frank Peabody and Lars Hansen played out a fun skit PAGE 2 Visitors and Announcements The show then transitioned beautifully 6 years: JJ Bronstein, Ed Ellis, Bob Elsner into recognition of an outstanding group 8 years: Frank Peabody 13 years: Ken Sherman 14 years: Helene Kalfuss 15 years: Dick Hostrop 17 years: Mike Brill 21 years: George Saulnier (in absentia) AND 37 YEARS! -- Milt Levinson of members with perfect attendance. -
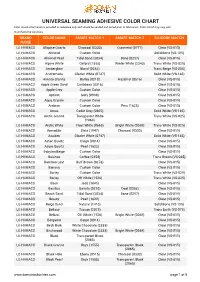
UNIVERSAL SEAMING ADHESIVE COLOR CHART Color Match Information Is Provided As Reference Only and Should Be Verifed and Tested Prior to Fabrication
LG HI- MACS UNIVERSAL SEAMING ADHESIVE COLOR CHART Color match information is provided as reference only and should be verifed and tested prior to fabrication. Color match may vary with manufacturing variations BRAND COLOR NAME SMART MATCH 1 SMART MATCH 2 SILICONE MATCH NAME LG HI-MACS Allspice Quartz Charcoal (0333) Gunmetal (0777) Clear (VS-015) LG HI-MACS Almond Custom Color Solid Bone (VS-185) LG HI-MACS Almond Pearl Tidal Sand (2034) Bone (0257) Clear (VS-015) LG HI-MACS Alpine White Oxford (1588) Winter White (2340) Trans White (VS-025) LG HI-MACS Amberglow Blond (0248) Trans Beige (VS-085) LG HI-MACS Andromeda Glacier White (0747) Solid White (VS-165) LG HI-MACS Annato Granite Barley (0218) Hazelnut (0816) Clear (VS-015) LG HI-MACS Apple Green Sand Caribbean (0316) Clear (VS-015) LG HI-MACS Apple Grey Custom Color Clear (VS-015) LG HI-MACS Apricot Ivory (0980) Clear (VS-015) LG HI-MACS Aqua Granite Custom Color Clear (VS-015) LG HI-MACS Arabian Custom Color Peru (1625) Clear (VS-015) LG HI-MACS Arcas Cadet Grey (0305) Solid White (VS-165) LG HI-MACS Arctic Granite Transparent White Trans White (VS-025) (2068) LG HI-MACS Arctic White Frost White (0665) Bright White (0268) Trans White (VS-025) LG HI-MACS Armadillo Slate (1947) Charcoal (0333) Clear (VS-015) LG HI-MACS Awaken Glacier White (0747) Solid White (VS-165) LG HI-MACS Aztec Quartz Cargo (0314) Clear (VS-015) LG HI-MACS Azure Quartz Pearl (1622) Clear (VS-015) LG HI-MACS Babylon Beige Custom Color Clear (VS-015) LG HI-MACS Balance Coffee (0354) Trans Brown (VS-065) LG HI-MACS -

Winter 2015 Cover Layout 1 11/12/15 5:20 PM Page 1 UCLA Basketball Ad 8.375X10.875-Bleed Layout 1 11/3/15 11:44 AM Page 1
winter 2015 cover_Layout 1 11/12/15 5:20 PM Page 1 UCLA Basketball Ad 8.375x10.875-Bleed_Layout 1 11/3/15 11:44 AM Page 1 CHAMPIONS BANK HERE #BetterBanking4Bruins 1-888-8WESCOM (1-888-893-7266) wescom.org 001 Insider's View_Layout 1 11/12/15 5:33 PM Page 1 BruIn BLue w i n t e r 2 0 1 5 THE INSIDER’S VIEW i n s i D e t H i s i s s u e his time a year ago, I sat down for lunch with the incomparable Rachel Robinson shortly after UCLA’s athletic and recreation facilities were named in honor of her late husband Jackie Robinson, the legendary four-sport Bruin star who went on InSIde THIS ISSue w i n t e r 2 0 1 5 to break Major League Baseball’s color barrier. T Rachel, who met Jackie at UCLA when uCLa aTHLeTICS In PHoToS ................2 / 6 / 10 / 14 both were students, reflected that afternoon, as you would expect, on her time here in SoarIng To neW HeIgHTS: russell westbrooK’s Journey Westwood. What you may not expect, from ucla to nba suPerstar ................................16 however, was how quickly the then 92-year- a Fa M I Ly a F Fa I r : fresHman aaron HoliDay old philanthropist, civil rights pioneer, mother of three, nurse with a NYU master’s becomes tHirD sibling to Don blue & golD ........22 degree and former assistant professor at Yale a reCIPe For SuCCeSS: JorDin canaDa & nirra fielDs moved the conversation to the exciting ProviDe unique cHemistry for women’s HooPs ....26 projects and initiatives that the Jackie CHaMPIonS Made Here ......................................30 Robinson Foundation, which she founded in 1973, had in the works. -

Media Kit (.Pdf)
CMMND is a Los Angeles-based art collective founded in 2018. Unlike traditional fashion brands or music groups, we strive to blur the lines between different artistic mediums. Through this approach, we utilize our individual skill sets to create collaborative projects ranging from seasonal apparel collections to a full length EP. Our mission is to promote the message that regardless of identity and career, everyone is a creative. This season CMMND became the youngest University of California licensee holder (Alongside the likes of Junya Watanabe [Fall 2019-UCLA] and Raf Simons [CK 205W39 Resort 2019-Cal]). Having been granted full access to the school’s 100 year old archive, we put our own contemporary spin on UCLA’s iconic marks like Retro Joe Bruin and Dunking Joe Bruin from the 70’s. Paying homage to throwback collegiate style, each piece is complemented with authentic details like raglan sleeves, vintage-inspired washes and chain stitch embroidery. With a foot both in the past and present, CMMND x UCLA captures our ongoing fascination with nostalgic fashion while creating a new gender-neutral uniform for today’s youth. Designed and produced in LA, this collection is crafted from locally milled organic cotton. “With many of my favorite local thrift stores closing down this year, I wanted this collection to encapsulate the thrill of discovering a one of a kind vintage college piece after hours of searching through bins and clothing racks”. - Bamidele Aleshe, Lead Designer Style: CU01AW20 Color: Powder Blue/UCLA Blue Material: 5 oz. Organic Jersey Description: Short sleeve t-shirt featuring a co-branded graphic of Retro Joe Bruin, UCLA’s classic mascot at left chest. -

Air Force Blue (Raf) {\Color{Airforceblueraf}\#5D8aa8
Air Force Blue (Raf) {\color{airforceblueraf}\#5d8aa8} #5d8aa8 Air Force Blue (Usaf) {\color{airforceblueusaf}\#00308f} #00308f Air Superiority Blue {\color{airsuperiorityblue}\#72a0c1} #72a0c1 Alabama Crimson {\color{alabamacrimson}\#a32638} #a32638 Alice Blue {\color{aliceblue}\#f0f8ff} #f0f8ff Alizarin Crimson {\color{alizarincrimson}\#e32636} #e32636 Alloy Orange {\color{alloyorange}\#c46210} #c46210 Almond {\color{almond}\#efdecd} #efdecd Amaranth {\color{amaranth}\#e52b50} #e52b50 Amber {\color{amber}\#ffbf00} #ffbf00 Amber (Sae/Ece) {\color{ambersaeece}\#ff7e00} #ff7e00 American Rose {\color{americanrose}\#ff033e} #ff033e Amethyst {\color{amethyst}\#9966cc} #9966cc Android Green {\color{androidgreen}\#a4c639} #a4c639 Anti-Flash White {\color{antiflashwhite}\#f2f3f4} #f2f3f4 Antique Brass {\color{antiquebrass}\#cd9575} #cd9575 Antique Fuchsia {\color{antiquefuchsia}\#915c83} #915c83 Antique Ruby {\color{antiqueruby}\#841b2d} #841b2d Antique White {\color{antiquewhite}\#faebd7} #faebd7 Ao (English) {\color{aoenglish}\#008000} #008000 Apple Green {\color{applegreen}\#8db600} #8db600 Apricot {\color{apricot}\#fbceb1} #fbceb1 Aqua {\color{aqua}\#00ffff} #00ffff Aquamarine {\color{aquamarine}\#7fffd4} #7fffd4 Army Green {\color{armygreen}\#4b5320} #4b5320 Arsenic {\color{arsenic}\#3b444b} #3b444b Arylide Yellow {\color{arylideyellow}\#e9d66b} #e9d66b Ash Grey {\color{ashgrey}\#b2beb5} #b2beb5 Asparagus {\color{asparagus}\#87a96b} #87a96b Atomic Tangerine {\color{atomictangerine}\#ff9966} #ff9966 Auburn {\color{auburn}\#a52a2a} #a52a2a Aureolin