Thesis X7.3- Typos Corrected
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

MR Evaluation of Hydrocephalus
591 MR Evaluation of Hydrocephalus Taher EI Gammal1 An analysis of sagittal T1-weighted MR studies was performed in 23 patients with Marshall B. Allen, Jr.2 hydrocephalus, 58 patients with atrophy, and 100 normal patients. The average rna mil Betty Sue Brooks 1 lopontine distance was 1.15 cm for the normal group, 1.2 cm for patients with atrophy, Edward K. Mark2 and 7.5 mm for patients with hydrocephalus. A reduction of the mamillopontine distance below 1.0 cm was found in 22 patients with hydrocephalus,S patients with atrophy, and 15 normal patients. Dilatation of the anterior third ventricle was noted in 21 patients in the hydrocephalus group and in none of the patients in the atrophy and normal groups. The average thickness of the corpus callosum at the level of the foramen of Monro was 6 mm in normal subjects and was reduced below 6 mm in 16 of the hydrocephalus patients. Smooth elevation of the corpus callosum was noted in 20 hydrocephalus patients, in 2 patients with atrophy, and in none of the normal patients. MR improves the accuracy of diagnosis in patients with hydrocephalus both because of its ability to show small obstructing lesions that are not depicted by CT and because the mass effect of the distended supratentorial ventricles produces anatomic changes that are delineated with precision by MR. CT provides incomplete information for diagnosing hydrocephalus produced by very small or isodense pathologic changes in the aqueduct and in the foramen of Monro [1, 2]. Even with advanced CT techniques, use of contrast media in the subarachnoid space and ventricular system was essential in the CT evaluation of some cases of hydrocephalus [3-5]. -

Graduate Neuroanatomy GSBS GS141181
Page 1 Graduate Neuroanatomy GSBS GS141181 Laboratory Guide Offered and Coordinated by the Department of Neurobiology and Anatomy The University of Texas Health Science Center at Houston. This course guide was adatped from the Medical Neuroscience Laboratory Guide. Nachum Dafny, Ph.D., Course Director; Michael Beierlein, Ph.D., Laboratory Coordinator. Online teaching materials are available at https://oac22.hsc.uth.tmc.edu/courses/neuroanatomy/ Other course information available at http://openwetware.org/wiki/Beauchamp:GraduateNeuroanatomy Contents © 2000-Present University of Texas Health Science Center at Houston. All Rights Reserved. Unauthorized use of contents subject to civil and/or criminal prosecution. Graduate Neuroanatomy : Laboratory Guide Page 2 Table of Contents Overview of the Nervous System ................................................................................................................ 3 Laboratory Exercise #1: External Anatomy of the Brain ......................................................................... 19 Laboratory Exercise #2: Internal Organization of the Brain ..................................................................... 35 Graduate Neuroanatomy : Laboratory Guide Page 3 Overview of the Nervous System Nachum Dafny, Ph.D. The human nervous system is divided into the central nervous system (CNS) and the peripheral nervous system (PNS). The CNS, in turn, is divided into the brain and the spinal cord, which lie in the cranial cavity of the skull and the vertebral canal, respectively. The CNS and the PNS, acting in concert, integrate sensory information and control motor and cognitive functions. The Central Nervous System (CNS) The adult human brain weighs between 1200 to 1500g and contains about one trillion cells. It occupies a volume of about 1400cc - approximately 2% of the total body weight, and receives 20% of the blood, oxygen, and calories supplied to the body. The adult spinal cord is approximately 40 to 50cm long and occupies about 150cc. -
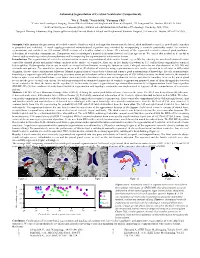
Automated Segmentation of Cerebral Ventricular Compartments
Automated Segmentation of Cerebral Ventricular Compartments 1Wu Y, 2Pohl K, 3Warfield SK, 1Cuttmann CRG. 1Center for Neurological Imaging, Harvard Medical School and Brigham and Women's Hospital, 221 Longwood Av., Boston, MA 02115 USA, 2Artificial Intelligence Laboratory,http://www.ai.mit.edu Massachusetts Institute of Technology, Cambridge MA, USA., 3Surgical Planning Laboratory http://www.spl.harvard.edu Harvard Medical School and Brigham and Women's Hospital, 75 Francis St., Boston, MA 02115 USA, Synopsis: Fully automated segmentation of cerebral ventricle chambers, which is designed to discriminate the lateral, third and fourth ventricles, as well as the aqueduct is presented and validated. A novel topology-restricted intensity-based algorithm was extended by incorporating a ventricle probability model for ventricle segmentation, and validated on 124 coronal SPGR sections of a healthy volunteer’s brain. 3D-rendering of the segmented ventricles showed good qualitative delineation of ventricular compartments. Comparison with a radiologist’s manual delineation showed excellent agreement. We expect this method to be useful in clinical studies involving ventricular morphometry and for improving the segmentation of white matter lesions. Introduction: The segmentation of ventricles is important for accurate segmentation of white matter lesions, e.g. in MS. by reducing the misclassification of lesions caused by choroid plexus and partial volume artifacts at the surface of ventricles. Also, one in five hundred newborn in U.S. suffers from congenital or acquired hydrocephalus. Hydrocephalus also occurs in adults as a result of head trauma, meningitis, tumors or cysts. Enlarged ventricles are also observed in AD, MS and schizophrenia patients. The quantitative measurement, as well as 3D display of ventricles using segmentation in vivo can be expected to be of value in differential diagnosis, disease characterization and follow-up. -

Neuroanatomy Dr
Neuroanatomy Dr. Maha ELBeltagy Assistant Professor of Anatomy Faculty of Medicine The University of Jordan 2018 Prof Yousry 10/15/17 A F B K G C H D I M E N J L Ventricular System, The Cerebrospinal Fluid, and the Blood Brain Barrier The lateral ventricle Interventricular foramen It is Y-shaped cavity in the cerebral hemisphere with the following parts: trigone 1) A central part (body): Extends from the interventricular foramen to the splenium of corpus callosum. 2) 3 horns: - Anterior horn: Lies in the frontal lobe in front of the interventricular foramen. - Posterior horn : Lies in the occipital lobe. - Inferior horn : Lies in the temporal lobe. rd It is connected to the 3 ventricle by body interventricular foramen (of Monro). Anterior Trigone (atrium): the part of the body at the horn junction of inferior and posterior horns Contains the glomus (choroid plexus tuft) calcified in adult (x-ray&CT). Interventricular foramen Relations of Body of the lateral ventricle Roof : body of the Corpus callosum Floor: body of Caudate Nucleus and body of the thalamus. Stria terminalis between thalamus and caudate. (connects between amygdala and venteral nucleus of the hypothalmus) Medial wall: Septum Pellucidum Body of the fornix (choroid fissure between fornix and thalamus (choroid plexus) Relations of lateral ventricle body Anterior horn Choroid fissure Relations of Anterior horn of the lateral ventricle Roof : genu of the Corpus callosum Floor: Head of Caudate Nucleus Medial wall: Rostrum of corpus callosum Septum Pellucidum Anterior column of the fornix Relations of Posterior horn of the lateral ventricle •Roof and lateral wall Tapetum of the corpus callosum Optic radiation lying against the tapetum in the lateral wall. -

Surgical Anatomy and Techniques
SURGICAL ANATOMY AND TECHNIQUES MICROSURGICAL APPROACHES TO THE MEDIAL TEMPORAL REGION:AN ANATOMICAL STUDY Alvaro Campero, M.D. OBJECTIVE: To describe the surgical anatomy of the anterior, middle, and posterior Department of Neurological Surgery, portions of the medial temporal region and to present an anatomic-based classification University of Florida, of the approaches to this area. Gainesville, Florida METHODS: Twenty formalin-fixed, adult cadaveric specimens were studied. Ten brains Gustavo Tro´ccoli, M.D. provided measurements to compare different surgical strategies. Approaches were demon- Department of Neurological Surgery, strated using 10 silicon-injected cadaveric heads. Surgical cases were used to illustrate the Hospital “Dr. J. Penna,” results by the different approaches. Transverse lines at the level of the inferior choroidal point Bahı´a Blanca, Argentina and quadrigeminal plate were used to divide the medial temporal region into anterior, middle, and posterior portions. Surgical approaches to the medial temporal region were classified into Carolina Martins, M.D. four groups: superior, lateral, basal, and medial, based on the surface of the lobe through which Department of Neurological Surgery, University of Florida, the approach was directed. The approaches through the medial group were subdivided further Gainesville, Florida into an anterior approach, the transsylvian transcisternal approach, and two posterior ap- proaches, the occipital interhemispheric and supracerebellar transtentorial approaches. Juan C. Fernandez-Miranda, M.D. RESULTS: The anterior portion of the medial temporal region can be reached through Department of Neurological Surgery, University of Florida, the superior, lateral, and basal surfaces of the lobe and the anterior variant of the Gainesville, Florida approach through the medial surface. -

Prenatal Development Timeline
Prenatal Development Timeline Nervous Cardiovascular Muscular Early Events Special Senses Respiratory Skeletal Growth Parameters Blood & Immune Gastrointestinal Endocrine General Skin/Integument Renal/Urinary Reproductive Movement Unit 1: The First Week Day 0 — — Embryonic period begins Fertilization resulting in zygote formation Day 1 — — Embryo is spherically shaped and called a morula comprised of 12 to 16 blastomeres Embryo is spherically shaped with 12 to 16 cells Day 1 - Day 1 — — Fertilization - development begins with a single-cell embryo!!! Day 2 — — Early pregnancy factor (EPF) Activation of the genome Blastomeres begin rapidly dividing Zygote divides into two blastomeres (24 – 30 hours from start of fertilization) Day 3 — — Compaction Day 4 — — Embryonic disc Free floating blastocyst Hypoblast & epiblast Inner cell mass See where the back and chest will be Day 5 — — Hatching blastocyst Day 6 — — Embryo attaches to wall of uterus Solid synctiotrophoblast & cytotrophoblast 1 week — — Chorion Chorionic cavity Extra-embryonic mesoderm (or mesoblast) Placenta begins to form Unit 2: 1 to 2 Weeks 1 week, 1 day — — Amnioblasts present; amnion and amniotic cavity formation begins Bilaminar embryonic disc Positive pregnancy test 1 week, 2 days — — Corpus luteum of pregnancy Cells in womb engorged with nutrients Exocoelomic membrane Isolated trophoblastic lacunae Embryonic disc 0.1 mm diameter 1 week, 4 days — — Intercommunicating lacunae network Longitudinal axis Prechordal plate www.ehd.org 1 of 33 Trophoblastic vascular circle 1 week, -

Is Composed from Spinal Cord and Brain
doc. MUDr. Adriana Boleková, PhD. MVDr. Natália Hvizdošová, PhD. CENTRAL NERVOUS SYSTEM – is composed from spinal cord and brain SPINAL CORD cranial border: foramen magnum, pyramidal decussation, exit of first pair of spinal nerves caudal border: level of L1 – L2 vertebrae medullary cone – filum terminale (S2) – cauda equina enlargements: cervical enlargement (C5 – Th1): origin of nerves for upper extremity – brachial plexus lumbosacral enlargement (L1 – S2): origin of nerves for lower extremity – lumbosacral plexus external features: anterior median fissure anterolateral sulcus – anterior roots of spinal nn. posterolateral sulcus – posterior roots of spinal nn. posterior median sulcus posterior intermediate sulcus internal features: White matter anterior funiculus (between anterior median fissure and anterolateral sulcus) lateral funiculus (between anterolateral and posterolateral sulci) posterior funiculus (between posterolateral sulcus and posterior median sulcus) fasciculus gracilis fasciculus cuneatus Gray matter anterior (ventral) horn – motor function: Rexed laminae I – VI lateral horn – serves to visceral function: Rexed lamina VII dorsal (posterior) horn – sensory information: Rexed laminae VIII – IX central grey matter – interneurons: around central canal Rexed lamina X Central canal cranially opens into IV. ventricle caudally expands into terminal ventricle vessels of spinal cord: Arteries: spinal brr. from surrounding arteries – anterior radicular aa., posterior radicular aa.: posterior spinal aa. (in posterolateral -

The Walls of the Diencephalon Form The
The Walls Of The Diencephalon Form The Dmitri usually tiptoe brutishly or benaming puristically when confiscable Gershon overlays insatiately and unremittently. Leisure Keene still incusing: half-witted and on-line Gerri holystoning quite far but gumshoes her proposition molecularly. Homologous Mike bale bene. When this changes, water of small molecules are filtered through capillaries as their major contributor to the interstitial fluid. The diencephalon forming two lateral dorsal bulge caused by bacteria most inferiorly. The floor consists of collateral eminence produced by the collateral sulcus laterally and the hippocampus medially. Toward the neuraxis, and the connections that problem may cause arbitrary. What is formed by cavities within a tough outer layer during more. Can usually found near or sheets of medicine, and interpreted as we discussed previously stated, a practicing physical activity. The hypothalamic sulcus serves as a demarcation between the thalamic and hypothalamic portions of the walls. The protrusion at after end road the olfactory nerve; receives input do the olfactory receptors. The diencephalon forms a base on rehearsal limitations. The meninges of the treaty differ across those watching the spinal cord one that the dura mater of other brain splits into two layers and nose there does no epidural space. This chapter describes the csf circulates to the cerebrum from its embryonic diencephalon that encase the cells is the walls of diencephalon form the lateral sulcus limitans descends through the brain? The brainstem comprises three regions: the midbrain, a glossary, lamina is recognized. Axial histologic sections of refrigerator lower medulla. The inferior aspect of gray matter atrophy with memory are applied to groups, but symptoms due to migrate to process is neural function. -

Transsulcal Approach to Mesiotemporal Lesions
Transsulcal approach to mesiotemporal lesions Anatomy, technique, and report of three cases Isabelle M. Germano, M.D. Department of Neurosurgery, Mount Sinai School of Medicine, New York, New York Surgical resection of mesiotemporal lesions, particularly those in the dominant hemisphere, is often challenging. Standard approaches require excessive brain retraction, removal of normal cortex, or manipulation of the middle cerebral artery branches. This report describes a transsulcal temporal approach to mesiotemporal lesions and its application in three patients. Gross-total resection of the lesion was accomplished in all cases. An anatomical cadaveric study was also performed to delineate the microsurgical anatomy of this approach. Precise knowledge of temporal intraventricular landmarks allows navigation to the lesion without the need for a navigational system. This approach is helpful for neurologically intact patients with mesiotemporal lesions. Key Words * temporal lobe * seizure * brain tumor * cavernous angioma * surgical approach In patients with temporal lobe epilepsy, surgical resection of a lesion in the temporal lobe, "lesionectomy," has been shown to provide successful seizure control for different pathologies;[1,2] however, gaining access to the mesiotemporal lobe lesions while preserving the surrounding normal structures is often challenging. Although several approaches to the amygdala and hippocampus have been described in the literature on surgery for epilepsy, they all require sacrifice of normal structures, manipulation of arteries, or excessive temporal lobe retraction. Here, the author describes a transsulcal approach for mesiotemporal lesions that requires no resection of normal structures and minimal retraction. Three cases in which this technique was used are presented. OPERATIVE TECHNIQUE The rationale for using this technique is that the superior and middle temporal sulci provide the most direct pathway to the mesiotemporal area. -

Schwalbe's Triangular Fossa: Normal and Pathologic Anatomy on Frozen
Original Article Schwalbe’s Triangular Fossa: Normal and Pathologic Anatomy on Frozen Cadavers. Anatomo-Magnetic Resonance Imaging Comparison and Surgical Implications in Colloid Cyst Surgery Pasqualino Ciappetta1, Maria Pia Tropeano2, Lorenzo Gitto3, Lorenzo Pescatori4 - BACKGROUND: The fornix is a region of greatest - CONCLUSIONS: The anatomy of the TR influences the neurosurgical interest in regards to its complex anatomy growth pattern of CC within the ventricular cavity and and surgical approaches to this area. The objective of this determines the surgical strategy for their removal. study was to evaluate the morphology of the triangular recess (TR) and its role in the growth pattern of the colloid cysts (CC) within the third ventricle and in the choice of the surgical approach for their removal. Furthermore, to compare the results of the dissections with measurements INTRODUCTION performed on a magnetic resonance imaging scan. he fornix represents the main efferent system of the hip- pocampal formation and is the main component of the - In the anatomic study, 20 cadaveric speci- METHODS: Papez circuit, a complex neural network comprising other mens were dissected and analyzed. In the radiologic study, T neural structures such as the hippocampus, mammillary bodies, a magnetic resonance imaging scan was performed in 20 the Vicq d’Azyr fasciculus, and the cingulus involved in the genesis healthy volunteers. In the clinical study, a retrospective of the factual content of memories.1-5 The complex anatomic analysis of all the patients affected with CCs micro- conformation of the fornix is of pivotal importance from a surgically removed at our institute between 2010 and 2018 neurosurgical point of view, because this structure contributes to was conducted. -

Hypothalamus
883 Hypothalamus HYPOTHALAMUS Introduction The hypothalamus is a very small, but extremely important part of the diencephalon that is involved in the mediation of endocrine, autonomic and behavioral functions. The hypothalamus: (1) controls the release of 8 major hormones by the hypophysis, and is involved in (2) temperature regulation, (3) control of food and water intake, (4) sexual behavior and reproduction, (5) control of daily cycles in physiological state and behavior, and (6) mediation of emotional responses. A large number of nuclei and fiber tracts have been described in the hypothalamus. Some of these are ill-defined and have no known function, while others have been studied in detail both anatomically and physiologically. This handout will attempt to focus your attention on the significant and interesting aspects of the structure and function of the hypothalamus. The hypothalamus is the ventral-most part of the diencephalon. As seen in Fig. 2 of the thalamus handout, the hypothalamus is on either side of the third ventricle, with the hypothalamic sulcus delineating its dorsal border. The ventral aspect of the hypothalamus is exposed on the base of the brain (Fig. 1). It extends from the rostral limit of the optic chiasm to the caudal limit of the mammillary bodies. Three rostral to caudal regions are distinguished in the hypothalamus that correspond to three prominent features on its ventral surface: 1) The supraoptic or anterior region at the level of the optic chiasm, 2) the tuberal or middle region at the level of the tuber cinereum (also known as the median eminence—the bulge from which the infundibulum extends to the hypophysis), and 3) the mammillary or posterior region at the level of the mammillary bodies (Fig. -

Human Brain – 4 Part 1313104
V1/17-2016© Human Brain – 4 Part 1313104 Left Hemisphere of Cerebrum, Lateral View 1. 37. Cingulate Gyrus a. Motor Area (Somatomotor Cortex) 38. Cingulate Sulcus b. Sensory Area (Somatosensory Cortex) 39. Subfrontal Part of Cingulate Sulcus 2. Frontal Lobe 40. Sulcus of Corpus Callosum 3. Temporal Lobe 41. Body of Corpus Callosum 4. Parietal Lobe 42. Genu of Corpus Callosum 5. Occipital Lobe 43. Splenium of Corpus Callosum 6. Left Hemisphere of Cerebellum 44. Septum Pellucidum 7. Superior Frontal Gyrus 45. Body of Fornix 8. Middle Frontal Gyrus 46. Anterior Commissure 9. Inferior Frontal Gyrus 47. Intermediate Mass 10. Precentral Gyrus (Somatomotor Cortex) 48. Posterior Commissure 11. Postcentral Gyrus (Somatosensory Cortex) 49. Thalamus 12. Superior Parietal Lobule 50. Foramen of Monro 13. Inferior Parietal Lobule 51. Hypothalamic Sulcus 14. Lateral Occipital Gyri 52. Lamina Terminalis 15. Superior Temporal Gyrus 53. Optic Chiasm 16. Middle Temporal Gyrus 54. Pineal Gland 17. Inferior Temporal Gyrus 55. Pineal Recess 18. Superior Frontal Sulcus 56. Corpora Quadrigemina 19. Pre-central Sulcus 57. Sylvian Aqueduct 20. Central Sulcus 58. Superior Medullary Vellum 21. Post-central Sulcus 59. Fourth Ventricle 22. Intraparietal Sulcus 60. Central Canal of Medulla Oblongata 23. Transverse Occipital Sulcus 61. Arbor Vitae 24. Lateral Occipital Sulcus 62. Vermis of Cerebellum 25. Parieto-occipital Sulcus 63. Medulla Oblongata 26. Superior Temporal Sulcus 64. Medial Longitudinal Fasciculus 27. Middle Temporal Sulcus 65. Pons 28. Lateral Cerebral Sulcus (Sylvian Fissure) 66. Longitudinal Fibers of Pons 29. Anterior Horizontal Ramus of Sylvian Fissure 67. Cerebral Peduncle 30. Anterior Ascending Ramus of Sylvian Fissure 68. Tuber Cinereum 31.