Occurrence of Arsenic in the Riverbed Sediments of The
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
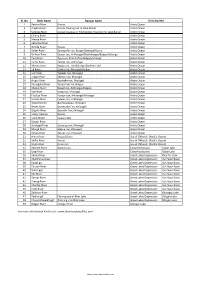
List of Rivers of Mongolia
Sl. No River Name Russian Name Draining Into 1 Yenisei River Russia Arctic Ocean 2 Angara River Russia, flowing out of Lake Baikal Arctic Ocean 3 Selenge River Сэлэнгэ мөрөн in Sükhbaatar, flowing into Lake Baikal Arctic Ocean 4 Chikoy River Arctic Ocean 5 Menza River Arctic Ocean 6 Katantsa River Arctic Ocean 7 Dzhida River Russia Arctic Ocean 8 Zelter River Зэлтэрийн гол, Bulgan/Selenge/Russia Arctic Ocean 9 Orkhon River Орхон гол, Arkhangai/Övörkhangai/Bulgan/Selenge Arctic Ocean 10 Tuul River Туул гол, Khentii/Töv/Bulgan/Selenge Arctic Ocean 11 Tamir River Тамир гол, Arkhangai Arctic Ocean 12 Kharaa River Хараа гол, Töv/Selenge/Darkhan-Uul Arctic Ocean 13 Eg River Эгийн гол, Khövsgöl/Bulgan Arctic Ocean 14 Üür River Үүрийн гол, Khövsgöl Arctic Ocean 15 Uilgan River Уйлган гол, Khövsgöl Arctic Ocean 16 Arigiin River Аригийн гол, Khövsgöl Arctic Ocean 17 Tarvagatai River Тарвагтай гол, Bulgan Arctic Ocean 18 Khanui River Хануй гол, Arkhangai/Bulgan Arctic Ocean 19 Ider River Идэр гол, Khövsgöl Arctic Ocean 20 Chuluut River Чулуут гол, Arkhangai/Khövsgöl Arctic Ocean 21 Suman River Суман гол, Arkhangai Arctic Ocean 22 Delgermörön Дэлгэрмөрөн, Khövsgöl Arctic Ocean 23 Beltes River Бэлтэсийн Гол, Khövsgöl Arctic Ocean 24 Bügsiin River Бүгсийн Гол, Khövsgöl Arctic Ocean 25 Lesser Yenisei Russia Arctic Ocean 26 Kyzyl-Khem Кызыл-Хем Arctic Ocean 27 Büsein River Arctic Ocean 28 Shishged River Шишгэд гол, Khövsgöl Arctic Ocean 29 Sharga River Шарга гол, Khövsgöl Arctic Ocean 30 Tengis River Тэнгис гол, Khövsgöl Arctic Ocean 31 Amur River Russia/China -

Journal Biology 2004 3
Mongolian Journal of Biological Sciences 2004 Vol. 2(1): 39-42 Hydrochemical Characteristics of Selenge River and its Tributaries on the Territory of Mongolia Bazarova J.G. 1, Dorzhieva S.G.1, Bazarov B.G. 1, Barkhutova D.D.2, Dagurova O.P.2, Namsaraev B.B. 2 and Zhargalova S.O.2 1Baikal Institute of Nature Management of SB RAS, Ulan-Ude, 2Institute of General and Experimental Biology of SB RAS, Ulan-Ude, E-mail: [email protected] Abstract Hydrochemical research of the Selenge and its main tributary the Orkhon river on the territory of Mongolia has been conducted. Concentrations of the main water ions were measured. Distribution of - + heavy metals was determined. Dynamics of biogenic elements (NO3 , NH4 , phosphates) and degree of phenol pollution was determined. Key words: Baikal, biogenic, heavy metals, ions, phenol, Selenge River Introduction river is polluted along its entire length. To assess the current ecological condition in the Mongolian During the last 30-40 years Lake Baikal has part of the Selenge river basin, it is necessary to been influenced by various anthropogenic factors. implement hydrobiological and hydrochemical Industrial and household waste water have changed monitoring. It requires information not only of the chemical composition of Baikal with a chemical composition, but of biogenic components deterioration in water quality in the basin territory. in the processes of accumulation and According to sustainable development policy, transformation in water, bottom sediments and river protection of Baikal region is considered -

Archaeological Investigations of Xiongnu Sites in the Tamir River
Archaeological Investigations of Xiongnu Sites in the Tamir River Valley Results of the 2005 Joint American-Mongolian Expedition to Tamiryn Ulaan Khoshuu, Ogii nuur, Arkhangai aimag, Mongolia David E. Purcell and Kimberly C. Spurr Flagstaff, Arizona (USA) During the summer of 2005 an archaeological investigations, and What is known points to this area archaeological expedition jointly their results, are the focus of this as one of the most important mounted by the Silkroad Foun- article, which is a preliminary and cultural regions in the world, a fact dation of Saratoga, California, incomplete record of the project recently recognized by the U.S.A. and the Mongolian National findings. Not all of the project data UNESCO through designation of University, Ulaanbataar, investi- — including osteological analysis of the Orkhon Valley as a World gated two sites near the the burials, descriptions or maps Heritage Site in 2004 (UNESCO confluence of the Tamir River with of the graves, or analyses of the 2006). Archaeological remains the Orkhon River in the Arkhangai artifacts — is available as of this indicate the region has been aimag of central Mongolia (Fig. 1). writing. Consequently, the greater occupied since the Paleolithic (circa The expedition was permitted emphasis falls on one of the two 750,000 years before present), (Registration Number 8, issued sites. It is hoped that through the with Neolithic sites found in great June 23, 2005) by the Ministry of Silkroad Foundation, the many numbers. As early as the Neolithic Education, Culture and Science of different collections from this period a pattern developed in Mongolia. The project had multiple project can be reunited in a which groups moved southward goals: archaeological investiga- scholarly publication. -

Tuul River Mongolia
HEALTHY RIVERS FOR ALL Tuul River Basin Report Card • 1 TUUL RIVER MONGOLIA BASIN HEALTH 2019 REPORT CARD Tuul River Basin Report Card • 2 TUUL RIVER BASIN: OVERVIEW The Tuul River headwaters begin in the Lower As of 2018, 1.45 million people were living within Khentii mountains of the Khan Khentii mountain the Tuul River basin, representing 46% of Mongolia’s range (48030’58.9” N, 108014’08.3” E). The river population, and more than 60% of the country’s flows southwest through the capital of Mongolia, GDP. Due to high levels of human migration into Ulaanbaatar, after which it eventually joins the the basin, land use change within the floodplains, Orkhon River in Orkhontuul soum where the Tuul lack of wastewater treatment within settled areas, River Basin ends (48056’55.1” N, 104047’53.2” E). The and gold mining in Zaamar soum of Tuv aimag and Orkhon River then joins the Selenge River to feed Burenkhangai soum of Bulgan aimag, the Tuul River Lake Baikal in the Russian Federation. The catchment has emerged as the most polluted river in Mongolia. area is approximately 50,000 km2, and the river itself These stressors, combined with a growing water is about 720 km long. Ulaanbaatar is approximately demand and changes in precipitation due to global 470 km upstream from where the Tuul River meets warming, have led to a scarcity of water and an the Orkhon River. interruption of river flow during the spring. The Tuul River basin includes a variety of landscapes Although much research has been conducted on the including mountain taiga and forest steppe in water quality and quantity of the Tuul River, there is the upper catchment, and predominantly steppe no uniform or consistent assessment on the state downstream of Ulaanbaatar City. -

DRAINAGE BASINS of the WHITE SEA, BARENTS SEA and KARA SEA Chapter 1
38 DRAINAGE BASINS OF THE WHITE SEA, BARENTS SEA AND KARA SEA Chapter 1 WHITE SEA, BARENTS SEA AND KARA SEA 39 41 OULANKA RIVER BASIN 42 TULOMA RIVER BASIN 44 JAKOBSELV RIVER BASIN 44 PAATSJOKI RIVER BASIN 45 LAKE INARI 47 NÄATAMÖ RIVER BASIN 47 TENO RIVER BASIN 49 YENISEY RIVER BASIN 51 OB RIVER BASIN Chapter 1 40 WHITE SEA, BARENT SEA AND KARA SEA This chapter deals with major transboundary rivers discharging into the White Sea, the Barents Sea and the Kara Sea and their major transboundary tributaries. It also includes lakes located within the basins of these seas. TRANSBOUNDARY WATERS IN THE BASINS OF THE BARENTS SEA, THE WHITE SEA AND THE KARA SEA Basin/sub-basin(s) Total area (km2) Recipient Riparian countries Lakes in the basin Oulanka …1 White Sea FI, RU … Kola Fjord > Tuloma 21,140 FI, RU … Barents Sea Jacobselv 400 Barents Sea NO, RU … Paatsjoki 18,403 Barents Sea FI, NO, RU Lake Inari Näätämö 2,962 Barents Sea FI, NO, RU … Teno 16,386 Barents Sea FI, NO … Yenisey 2,580,000 Kara Sea MN, RU … Lake Baikal > - Selenga 447,000 Angara > Yenisey > MN, RU Kara Sea Ob 2,972,493 Kara Sea CN, KZ, MN, RU - Irtysh 1,643,000 Ob CN, KZ, MN, RU - Tobol 426,000 Irtysh KZ, RU - Ishim 176,000 Irtysh KZ, RU 1 5,566 km2 to Lake Paanajärvi and 18,800 km2 to the White Sea. Chapter 1 WHITE SEA, BARENTS SEA AND KARA SEA 41 OULANKA RIVER BASIN1 Finland (upstream country) and the Russian Federation (downstream country) share the basin of the Oulanka River. -

The Linkage of the Precipitation in the Selenga River Basin to Midsummer Atmospheric Blocking
atmosphere Article The Linkage of the Precipitation in the Selenga River Basin to Midsummer Atmospheric Blocking Olga Yu. Antokhina 1,*, Pavel N. Antokhin 1 , Yuliya V. Martynova 2,3 and Vladimir I. Mordvinov 4 1 Zuev Institute of Atmospheric Optics SB RAS, 634021 Tomsk, Russia; [email protected] 2 Institute of Monitoring of Climatic and Ecological Systems SB RAS, 634055 Tomsk, Russia; [email protected] 3 Siberian Regional Hydrometeorological Research Institute, 630099 Novosibirsk, Russia 4 Institute of Solar-Terrestrial Physics, SB RAS, 664033 Irkutsk, Russia; [email protected] * Correspondence: [email protected]; Tel.: +7-952-154-4669 Received: 3 June 2019; Accepted: 21 June 2019; Published: 24 June 2019 Abstract: The linkage between atmospheric blocking (blocking frequency, BF) and total monthly July precipitation in the Selenga River Basin, the main tributary of Lake Baikal, for the period 1979–2016 was investigated. Based on empirical orthogonal functions (EOF) analysis, two dominant modes of precipitation over the Selenga River Basin were extracted. The first EOF mode (EOF 1) is related to precipitation fluctuations mainly in the Mongolian part of Selenga; the second EOF mode (EOF 2)—in the Russian part of Selenga. Based on two different modes obtained, the total amount of precipitation individually for the Russian and Mongolian part of Selenga was calculated. Correlation analysis has demonstrated that precipitation over the Mongolian part of the Selenga Basin is positively correlated to blocking over Eastern Siberia/Mongolia (80–120◦ E, ESM-BF). Precipitation over the Russian part of the Selenga Basin is positively correlated to blocking over the Urals-Western Siberia (50–80◦ E, UWS-BF) and European blocking (0–50◦ E, E-BF). -

Thesis Local Understanding of Hydro-Climate Changes
THESIS LOCAL UNDERSTANDING OF HYDRO-CLIMATE CHANGES IN MONGOLIA Submitted by Tumenjargal Sukh Department of Ecosystem Science and Sustainability In partial fulfillment of the requirements For the Degree of Master of Science Colorado State University Fort Collins, Colorado Fall 2012 Master’s Committee: Advisor: Steven Fassnacht Melinda Laituri Maria Fernandez-Gimenez Greg Butters Copyright by Tumenjargal Sukh 2012 All Rights Reserved ABSTRACT LOCAL UNDERSTANDING OF HYDRO-CLIMATE CHANGES IN MONGOLIA Air temperatures have increased more in semi-arid regions than in many other parts of the world. Mongolia has an arid/semi-arid climate where much of the population is dependent upon the limited water resources, especially herders. This paper combines herder observations of changes in water availability in streams and from groundwater with an analysis of climatic and hydrologic change from station data to illustrate the degree of change of Mongolian water resources. We find that herders’ local knowledge of hydro-climatic changes is similar to the station based analysis. However, station data are spatially limited, so local knowledge can provide finer scale information on climate and hydrology. We focus on two regions in central Mongolia: the Jinst soum in Bayankhongor aimag in the desert steppe region and the Ikh-Tamir soum in Arkhangai aimag in the mountain steppe. As the temperatures have increased significantly (more in Ikh-Tamir than Jinst), precipitation amounts have decreased in Ikh-Tamir which corresponds to a decrease in streamflow, in particular, the average annual streamflow and the annual peak discharge. At Erdenemandal (Ikh-Tamir) the number of days with precipitation has decreased while at Horiult (Jinst) it has increased. -

Metadata Describing the Kharaa Yeröö River Basin Water Quality Database
Metadata describing the Kharaa Yeröö River Basin Water Quality Database Jürgen Hofmann , Ralf Ibisch, Daniel Karthe , Buren Scharaw, Michael Schäffer, Melanie Hartwig, Philipp Theuring , Michael Rode, Saulyegul Avlyush , Vanessa Watson, Vanessa Bremerich , Gerel Osor, Andrew Kaus , Katja Westphal, Martin Pfeiffer , Jörg Priess , Christian Schweitzer, Daniel Krätz, Jonas Gröning , Jens Hürdler, Gunsmaa Batbayar , Sonja Heldt, Olaf Büttner & Dietrich Borchardt Freshwater Metadata Journal DOI 10.15504/fmj.2018.36 Published by University of Natural Resources and ISSN 2312-6604 Life Sciences, Institute of Hydrobiology and Published online: 2018-09-28 Aquatic Ecosystem Management, BOKU - Vienna Freshwater Metadata Journal 36: 1-10 DOI 10.15504/fmj.2018.36 http://freshwaterjournal.eu ISSN 2312-6604 Metadata describing the Kharaa Yeröö River Basin Water Quality Database Jürgen Hofmann 1 , Ralf Ibisch 4, Daniel Karthe 7 , Buren Scharaw 5, Michael Schäffer 4, Melanie Hartwig 4, Philipp Theuring 4 , Michael Rode 3, Saulyegul Avlyush 9 , Vanessa Watson 6, Vanessa Bremerich 1 , Gerel Osor 8, Andrew Kaus 4 , Katja Westphal 3, Martin Pfeiffer 3 , Jörg Priess 3 , Christian Schweitzer 4, Daniel Krätz 4, Jonas Gröning 1 , Jens Hürdler 2, Gunsmaa Batbayar 7 , Sonja Heldt 10, Olaf Büttner 3 & Dietrich Borchardt 3 1 Leibniz-Institute of Freshwater Ecology and Inland Fisheries (IGB), Berlin, Germany; corresponding author: [email protected] 2 formerly: Leibniz-Institute of Freshwater Ecology and Inland Fisheries (IGB), Berlin, Germany 3 Helmholtz-Centre -

Subject of the Russian Federation)
How to use the Atlas The Atlas has two map sections The Main Section shows the location of Russia’s intact forest landscapes. The Thematic Section shows their tree species composition in two different ways. The legend is placed at the beginning of each set of maps. If you are looking for an area near a town or village Go to the Index on page 153 and find the alphabetical list of settlements by English name. The Cyrillic name is also given along with the map page number and coordinates (latitude and longitude) where it can be found. Capitals of regions and districts (raiony) are listed along with many other settlements, but only in the vicinity of intact forest landscapes. The reader should not expect to see a city like Moscow listed. Villages that are insufficiently known or very small are not listed and appear on the map only as nameless dots. If you are looking for an administrative region Go to the Index on page 185 and find the list of administrative regions. The numbers refer to the map on the inside back cover. Having found the region on this map, the reader will know which index map to use to search further. If you are looking for the big picture Go to the overview map on page 35. This map shows all of Russia’s Intact Forest Landscapes, along with the borders and Roman numerals of the five index maps. If you are looking for a certain part of Russia Find the appropriate index map. These show the borders of the detailed maps for different parts of the country. -

Observed Trends of Climate and River Discharge in Mongolia's Selenga
water Article Observed Trends of Climate and River Discharge in Mongolia’s Selenga Sub-Basin of the Lake Baikal Basin Batsuren Dorjsuren 1,2 , Denghua Yan 1,3,4,*, Hao Wang 1,3,4, Sonomdagva Chonokhuu 2 , Altanbold Enkhbold 5, Xu Yiran 6, Abel Girma 1,7 , Mohammed Gedefaw 1,7 and Asaminew Abiyu 1 1 College of Environmental Science and Engineering, Donghua University, Shanghai 201620, China; [email protected] (B.D.); [email protected] (H.W.); [email protected] (A.G.); [email protected] (M.G.); [email protected] (A.A.) 2 Department of Environment and Forest Engineering, School of Engineering and Applied Sciences, National University of Mongolia, Ulaanbaatar 210646, Mongolia; [email protected] 3 State Key Laboratory of Simulation and Regulation of Water Cycle in River Basin, China Institute of Water Resources and Hydropower Research (IWHR), Beijing 100038, China 4 Water Resources Department, China Institute of Water Resources and Hydropower Research (IWHR), Beijing 100038, China 5 Department of Geography, School of Art & Sciences, National University of Mongolia, Ulaanbaatar 210646, Mongolia; [email protected] 6 School of Resources and Earth Science, China University of Mining and Technology, Xuzhou 221116, China; [email protected] 7 Department of Natural Resource Management, University of Gondar, Gondar 196, Ethiopia * Correspondence: [email protected]; Tel.: +86-10-68781976 Received: 11 September 2018; Accepted: 9 October 2018; Published: 12 October 2018 Abstract: Mongolia’s Selenga sub-basin of the Lake Baikal basin is spatially extensive, with pronounced environmental gradients driven primarily by precipitation and air temperature on broad scales. Therefore, it is an ideal region to examine the dynamics of the climate and the hydrological system. -

Baikal Project 2012-2014 Results and Events Booklet.Pdf
Photo by Elena Chumak GEF: “The GEF unites 182 countries in partnership with international institutions, non-governmental organizations (NGOs), and the private sector to address global environmental issues while supporting national sustainable development initiatives. Today the GEF is the largest public funder of projects to improve the global environment. An independently operating financial organization, the GEF provides grants for projects related to biodiversity, climate change, international waters, land degradation, the ozone layer, and persistent organic pollutants. Since 1991, GEF has achieved a strong track record with developing countries and countries with economies in transition, providing $9.2 billion in grants and leveraging $40 billion in co-financing for over 2,700 projects in over 168 countries. www.thegef.org” UNDP: “UNDP partners with people at all levels of society to help build nations that can withstand crisis, and drive and sustain the kind of growth that improves the quality of life for everyone. On the ground in 177 countries and territories, we offer global perspective and local insight to help empower lives and build resilient nations. www.undp.org” UNOPS: is an operational arm of the United Nations, helping a range of partners implement $1 billion worth of aid and development projects every year. UNOPS mission is to expand the capacity of the UN system and its partners to implement peacebuilding, humanitarian and development operations that matter for people in need. Photo by Elena Chumak Contents Project Achievements -

Modelling Land-Use and Land-Cover Change and Related Environmental Impacts in Northern Mongolia
Modelling land-use and land-cover change and related environmental impacts in Northern Mongolia Dissertation Zur Erlangung des akademischen Grades doctor rerum naturalium (Dr. rer. nat.) vorgelegt der Naturwissenschaftlichen Fakultät III der Martin-Luther-Universität Halle-Wittenberg von Christian Schweitzer geboren am 08.12.1977 in Frankenberg (Eder) Gutachter: 1. Prof. Dr. Ralf Seppelt 2. Prof. Dr. Wolfgang Cramer Halle (Saale), 12.04.2012 (Datum der Verteidigung) ‘The only progress that knowledge allows is in enabling us to describe more and more in detail the world we see and its evolution. What matters in a world-view is to grasp the meaning and purpose of everything, and that we cannot do. ’ Albert Schweitzer Acknowledgements The thesis presented here is the result of almost four years of scientific research conducted at the Center for Environmental Systems Research (CESR), University Kassel and at the Department Computational Landscape Ecology (CLE) at the Helmholtz Centre for Environmental Research in Leipzig. The work was funded by the German Federal Ministry for Education and Research (BMBF). It is a pleasure to thank those who made this thesis possible. I am grateful to Prof. Dr. Joseph Alcamo for his input during the first phase of this work. Further, it’s a great pleasure to thank Prof. Dr. Ralf Seppelt for supervising this thesis at CLE. I owe my deepest gratitude to my immediate supervisor Dr. Jörg A. Priess for supporting and teaching me during the last years. I thank him especially for introducing me to the topic of land-change science and environmental modelling and further for the countless hours of scientific discussions.