19 September 2019
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Emergency Care Weekly Metadata
Publication Metadata (including revision details) Metadata Description Indicator Publication Weekly Update of Emergency Department Activity and Waiting title Times. Description This publication reports key statistics on attendances at Emergency Departments (ED) across Scotland. The information presented in the publication includes trends in the number of attendances and length of time patients spend in ED. Theme Health and Social Care Topic Emergency Care Format Webpage, Excel workbook and CSV. Data source(s) NHS Board aggregate submissions to PHS on Emergency Department Activity and Waiting Times. Date that data Tuesday of the week prior to publication are acquired Release date Every Tuesday Frequency Weekly Timeframe of New data for the week ending 9 days before publication (e.g. 16 data and April publication contains data for week ending 7 April) timeliness Continuity of 1) A&E discharge times at hospitals in NHS Lothian were not data accurately recorded up to November 2017. The Academy of Medical Royal Colleges was commissioned by Scottish Government to ascertain the causes for the data issues in NHS Lothian. The review findings were published 26 June 2018. 2) Since 3 March 2015, the Scottish Government (SG) has released Official Statistics weekly A&E activity and waiting times information for the EDs in Scotland, derived from aggregate information supplied by NHS Boards on the number of attendances and 4, 8, and 12 hour waits. PHS (formally ISD) took over this data collection for statistics covering the week ending 7 June 2015. 3) From 20 May 2018, Raigmore hospital in NHS Highland trialled a new patient flow system. As a consequence the accuracy of some patients’ waits may have been affected between this date and 4 July, however the total number of attendances remains correct. -

NHS Guidlines
NHSScotland Identity guidelines Identikit Introduction In December 2000, Susan Deacon MSP, In this publication, the Minister said: “The public relate to and recognise Minister for Health and Community Care, the NHS. They believe their care is launched ‘Our National Health: provided by a national health service and staff take pride in the fact that a plan for action, a plan for change’ they work for the NHS. Research tells us that the variety of differently which set out a clear direction for the NHS named NHS bodies confuses the in Scotland with the aims of improving public and alienates staff. As part of our proposals to rebuild the National people’s health and creating a 21st century Health Service we will promote a new identity for the NHS in Scotland.” health service. The guidelines that follow provide an essential design toolkit to establish “Alongside the changes in NHS this new identity. The guidelines cover signage, vehicles, uniforms, stationery, boardrooms, we will re-establish literature, forms and other items. The a national identity for the aim is to replace, over time, the array of existing identities within NHS NHS in Scotland.” organisations with the single NHS identity while avoiding wastage and unnecessary expenditure. Our National Health: a plan for action, a plan for change section 3/page 31 2 Contents Section 1 Our national identity 4 Exclusion zone 6 Minimum size 6 Section 2 Identity structure 7 Essential elements 9 Identity variants 10 Caring device 12 Positioning the identity 14 Other identities 15 Working in partnership 16 Section 3 Identities for ideas & initiatives 17 Initiatives 18 Section 4 NHSScotland typefaces 19 Stone Sans 20 Arial 24 Garamond 25 Times New Roman 26 Literature 27 Section 5 Colour 28 Using colour 29 Primary colours 30 Colour palette 31 Tints 32 Printing the identity 33 3 Section One Our national identity Together, the initials ‘NHS’ and the caring symbol form the foundations of our identity. -

NHS Western Isles Written Submission
HS/S5/18/16/7 Submission for the Scottish Parliament Health and Sport Committee 15 May 2018 Gordon Jamieson Chief Executive, NHS Western Isles Page 1 of 329 HS/S5/18/16/7 NHS WESTERN ISLES LOCAL DELIVERY PLAN 2017/18 Filename LDP Version 2 Owner Dr Maggie Watts Director of Public Health Author Michelle McPhail Business Manager 1 Page 2 of 329 HS/S5/18/16/7 CONTENT Strategic Priority 1 – Health Inequalities and Prevention 1.1 ~ NHS Procurement Policies 1.2 ~ Employment policies supporting people to gain employment 1.3 ~ Supporting staff to support most vulnerable populations 1.4 ~ Using Horticulture as a complementary form of therapy 1.5 ~ Smoke Hebrides 1.6 ~ Health Promoting Health Service 1.7 ~ Healthy Working Lives 1.8 ~ Alcohol 1.9 ~ Obesity / Weight management 1.10 ~ Detect Cancer Early 1.11 ~ Police custody healthcare referral pathways Strategic Priority 2 – Antenatal and Early Years 2.1 ~ Duties consequent to Children and Young People (Scotland) Act 2014 – Staff development 2.2 ~ Health Visiting Strategic Priority 3 – Person Centred Care 3.1 ~ Person centred care (“Must do with Me”) 3.2 ~ Staff and public feedback 3.3 ~ Feedback and complaints – closing the loop Strategic Priority 4 – Safe Care 4.1 ~ Excellence in Care 4.2 ~ Scottish Patient Safety Programme rollout of acute programme into primary care, maternity, neonates and paediatrics and mental health services Strategic Priority 5 – Primary Care 5.1 ~ Strategic Intentions – 5.1.1 ~ Leadership and workforce 5.1.2 ~ Prioritised local actions to increase capacity 5.1.3 ~ Technology -
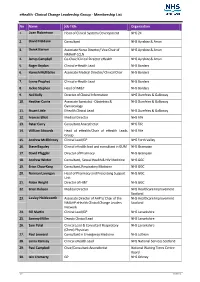
CCLG – Membership List
eHealth -Clinical Change Leadership Group - Membership List No Name Job Title Organisation 1. Joan Robertson Head of Clinical Systems Development NHS 24 2. David Haldane Consultant NHS Ayrshire & Arran 3. Derek Barron Associate Nurse Director/ Vice Chair of NHS Ayrshire & Arran NMAHP CCLN 4. James Campbell Co-Chair/Clinical Director eHealth NHS Ayrshire & Arran 5. Roger Brydon Clinical e-Health Lead NHS Borders 6. Hamish McRitchie Associate Medical Director/ Clinical Chair NHS Borders 7. Lynne Prophet Clinical e-Health Lead NHS Borders 8. Jackie Stephen Head of IM&T NHS Borders 9. Neil Kelly Director of Clinical Information NHS Dumfries & Galloway 10. Heather Currie Associate Specialist - Obstetrics & NHS Dumfries & Galloway Gynaecology 11. Stuart Little EHealth Clinical Lead NHS Dumfries & Galloway 12. Frances Elliot Medical Director NHS Fife 13. Peter Curry Consultant Anaesthetist NHS Fife 14. William Edwards Head of eHealth/Chair of eHealth Leads NHS Fife Group 15. Andrew McElhinney Clinical Lead/GP NHS Forth Valley 16. Steve Baguley Clinical eHealth lead and consultant in GUM NHS Grampian 17. David Pfeggler Director of Pharmacy NHS Grampian 18. Andrew Winter Consultant, Sexual Health & HIV Medicine NHS GGC 19. Brian Choo-Kang Consultant, Respiratory Medicine NHS GGC 20. Norman Lannigan Head of Pharmacy and Prescribing Support NHS GGC Unit 21. Robin Wright Director of HI&T NHS GGC 22. Brian Robson Medical Director NHS Healthcare Improvement Scotland 23. Lesley Holdsworth Associate Director of AHP’s/ Chair of the NHS Healthcare Improvement NMAHP eHealth Clinical Change Leaders Scotland Network 24. Bill Martin Clinical Lead/GP NHS Lanarkshire 25. Sammy Miller Deputy Clinical Lead NHS Lanarkshire 26. -

Bimonthly Report – July 2019 the Following Healthcare Associated Infect
Board Meeting NHS GRAMPIAN 03 09 19 Open Session Healthcare Associated Infection (HAI) Item 14 Bimonthly Report – July 2019 The following Healthcare Associated Infection Reporting Template (HAIRT) report contains NHS Grampian’s surveillance data and associated infection rates as reported in Health Protection Scotland’s (HPS) Quarterly Epidemiological Data for Quarter 1 (January to March 2019) published on 2 nd July 2019. 1 HAI Report July 2019 Additional Surveillance not reported in Health Protection Scotland’s Quarterly Epidemiological report: Methicillin-Resistant Staphylococcus Aureus (MRSA) Screening MRSA (CRA) screening compliance for Quarter 4 (January – March 2019) was 87% which is below the compliance target of 90% but above the national average (83%). Carbapenemase Producing Enterobacteriaceae (CPE) Screening CPE (CRA) screening compliance for Quarter 4 (January – March 2019) was 97% which is above both the compliance target (90%) and the national average (81%). Norovirus For the period April – June 2019 there were 4 wards closed (either completely or partially) in NHS Grampian due to enteric illness (confirmed or suspected Norovirus). Health Facilities Scotland (HFS) The cleaning compliance for April – June 2019 was 93% and the estates monitoring compliance was 94%; both these scores are above the national targets of 90%. 2 HAI Report July 2019 1. Actions Recommended The Board is requested to note the content of this summary bimonthly HAI Report, as directed by the HAI Policy Unit, Scottish Government Health Directorates. 2. Strategic Context • National Hospital Antimicrobial Prescribing Quality Indicators for 2017-18 • Local Delivery Plan Standards for CDIs & SABs awaited from Scottish Government • National Key Performance Indicators for MRSA screening • National Key Performance Indicators for CPE screening • National Health Facilities Scotland (HFS) Environmental Cleaning Target • National Health Facilities Scotland (HFS) Estates Monitoring Target • National Hand Hygiene Compliance Target 3 HAI Report July 2019 3. -

Scotland's Baby
Scotland’s Baby Box 5th March 2018 Cards Boxes Health Board 19/6-2/3 Received Delivered NHS Ayrshire and Arran 2279 1830 NHS Borders 728 580 NHS Dumfries and Galloway 869 719 NHS Fife 2304 1857 NHS Forth Valley 1891 1564 NHS Grampian 4131 3318 NHS Greater Glasgow and Clyde 9164 7410 NHS Highland 1800 1459 NHS Lanarkshire 3964 3234 NHS Lothian 6399 5182 NHS Orkney 131 110 NHS Shetland 144 115 NHS Tayside 2548 2140 NHS Western Isles 137 110 Total 36489 29628 WC 29/1/18 WC 5/2/18 WC 12/2/18 WC 19/2/18 WC 26/2/18 Cards Received 1049 988 997 1020 663 Orders Confirmed 1187 912 909 941 550 Orders On Hold 80 46 62 49 38 Total Received 19/6-2/3 37152 Total Confirmed 19/6-2/3 35662 Due date by Month 19/6-2/3 Aug-17 2171 Sep-17 3908 Oct-17 3897 Nov-17 3772 Dec-17 3886 Jan-18 3892 Feb-18 3477 Mar-18 3541 Apr-18 3173 May-18 2591 Jun-18 1181 Jul-18 114 Aug-18 1 Total 35604 19/6-2/3 Total Contact for Research 9500 Total Parentclub 8232 Boxes Delivered 29628 Cards Received/Delivered by HB 19/6-2/3 NHS Western Isles 110137 2140 NHS Tayside 2548 NHS Shetland 115 144 NHS Orkney 110 131 5182 NHS Lothian 6399 3234 NHS Lanarkshire 3964 1459 NHS Highland 1800 Boxes Delivered 7410 NHS Greater Glasgow and Clyde 9164 3318 NHS Grampian 4131 Cards Received 1564 NHS Forth Valley 1891 1857 NHS Fife 2304 719 NHS Dumfries and Galloway 869 580 NHS Borders 728 1830 NHS Ayrshire and Arran 2279 0 2000 4000 6000 8000 10000 BABYBOX ORDERS 1400 1200 1187 1049 1020 988 997 1000 912 909 941 800 663 600 550 400 200 80 62 46 49 38 0 WC 29/1/18 WC 5/2/18 WC 12/2/18 WC 19/2/18 WC -

NHS Western Isles Annual Report and Accounts 2011/12 2 NHS Western Isles Annual Report 2011/12 NHS Western Isles Annual Report 2011/12
NHS Western Isles Annual Report and Accounts 2011/12 NHS Western Isles Annual Report 2011/12 2 NHS Western Isles Annual Report 2011/12 Maggie Fraser NHS Western Isles 37 South Beach Stornoway Isle of Lewis HS1 2BB Tel: 01851 708060 Email: [email protected] If you wish to have sections of this document reproduced in another format or language, please contact the above address. Gaelic: Ma thogras sibh earrainean dhe'n sgrìobhainn seo a bhith air a mhac-shamhlachadh ann an cruth neo cànan eile, cuiribh fios, ma 'se bhur toil e, do'n seòladh gu h-àrd Polish: Na życzenie fragmenty tej informacji mogą być powielone w innym formacie lub języku, proszę o kontakt na dane podane powyżej Portuguese: Se desejar receber partes da presente informação reproduzidas noutro formato ou idioma, utilize os detalhes de contacto supramencionados Latvian: Ja Jūs vēlaties saņemt šīs informācijas daļas, reproducētas citā formātā vai valodā, Lūdzu, lietojiet augstāk minēto kontaktinformāciju Lithuanian: Jei jūs norėtumėte, jog ši informacija būtų pateikta kitu formatu ar kita kalba, prašome susisiekti viršuje pateiktais kontaktais Russian: Если Вы хотите получить какую-либо из этой информации в другом формате или на другом языке, пожалуйста, воспользуйтесь вышеуказанными контактными данными Czech: Pokud chcete některé z informací reprodukovat v jiném formátu nebo jazyce, obraťte se na nás na níže uvedeném kontaktu Bengali: NHS Western Isles Annual Report 2011/12 NHS Western Isles Annual Report 2011/12 3 Contents Page Introduction Page 4 Foreword by the Chair, -

Psychological Therapies Waiting Times in NHS Scotland – Report Publication
Psychological Therapies Waiting Times in NHS Scotland – Report Publication Quarter Ending 31 March 2021 Publication date: 1 June 2021 An Official Statistics release for Scotland Public Health Scotland This is an Official Statistics publication The Official Statistics (Scotland) Order 2008 authorises Public Health Scotland (formerly NHS National Services Scotland (the legal name being the Common Services Agency for the Scottish Health Service)) to produce official statistics. All official statistics should comply with the UK Statistics Authority’s Code of Practice which promotes the production and dissemination of official statistics that inform decision making. They can be formally assessed by the UK Statistics Authority’s regulatory arm for National Statistics status. Find out more about the Code of Practice at: https://www.statisticsauthority.gov.uk/osr/code-of-practice/ Find out more about official statistics at: https://www.statisticsauthority.gov.uk/national-statistician/producers-of-official-statistics/ 2 Public Health Scotland Contents Introduction .............................................................................................................................. 4 Main points ............................................................................................................................... 6 How long people waited to start their treatment (Patients Seen) .......................................... 7 People waiting for treatment at the end of the quarter ....................................................... -

North of Scotland Facilities & Capital Planning Group
Issue 1 3 September 2019 North of Scotland Facilities & Capital Planning Group Welcome to our First Edition of the North of Scotland’s news update for Facilities & Capital Planning Meet the team………. Chair/Programme Sponsor Paul Allen – Director of Facilities & eHealth, NHS Grampian Regional Leads Billy Alexander – Head of Soft Facilities, NHS Tayside Mark Anderson – Head of Property, NHS Tayside Lawson Bisset – Head of Facilities & Estates, NHS Shetland Malcolm Colquhoun – Head of Hospital & Support Services, NHS Orkney Gerry Donald – Head of Property & Asset Development, NHS Grampian Eric Green – Head of Estates, NHS Highland Douglas MacKenzie - Estates Manager, NHS Western Isles Gavin Payne – General Manager, Facilities & Estates, NHS Grampian Alistair Wilson – Professional Lead, Soft Services, NHS Highland Programme Manager Jill Beattie—NoS Programme Manager Facilities & Capital Planning “Our mission is to work in collaboration with the North of Scotland Health Boards to Read on to find out what the North deliver a set of Improvement Projects and of Scotland Facilities and Capital workstreams that will Planning Group have done over deliver measureable benefits for recent months. Facilities” Paul Allen, Programme Sponsor North of Scotland Facilities & Capital Planning Group Planning Workshop A very successful planning workshop of priority workstreams based on was held in Aberdeen on 26th June the anticipated benefits and 2019. Representatives from the potential impact on the North North Boards participated in Region. discussions to review the progress Eight improvement projects were and developments to date and identified as delivering the greatest collaboratively agree the priorities for potential benefits and further detail the future. The existing workplan was is provided below. -

1 NHS GRAMPIAN North of Scotland – Memorandum of Understanding 1
Board Meeting 02 08 18 Open Session NHS GRAMPIAN Item 10 North of Scotland – Memorandum of Understanding 1. Actions Recommended The Board is asked to: Consider the initial Memorandum of Understanding (MOU) document in relation to staff working across the North of Scotland (NoS) Health Boards. Delegate authority to the Chief Executive/Acting Chief Executive to sign off the MOU. 2. Strategic Context This report contributes to the North of Scotland (NoS) Regional Development Plan and NoS Workforce Programme Objectives. 3. Key matters relevant to recommendation The NoS Chief Executives’ meeting in February 2018 agreed, in principle, to support the development of an MOU (Appendix 1) to support staff to deliver services across NHS Board boundaries in the North of Scotland. This requirement was highlighted during the engagement related to the preparation of the North of Scotland Health and Social Care Delivery Plan, given the need to provide mutual support for services across the North, and the benefits associated with clinicians participating in regional clinical networks. When the Board considered this proposal at its meeting on 26th June 2018, the Board sought further information until clarity was sought regarding the penultimate paragraph in the Principles section. This paragraph reads: “In order to facilitate ease of staff working across the North of Scotland Boards in the future, the Partners intend that future employees are appointed on a contract of employment which includes regional working across the North of Scotland Boards as a requirement of their role.” There was concern that the intention would be to apply this to all staff contracts and be used to facilitate movement of large groups of staff to other Boards. -

Everyone Matters Pulse Survey National Report 2020
Health and Social Care Everyone Matters Pulse Survey National Report 2020 Page 0 of 91 Everyone Matters Pulse Survey 2020 National Report for Health and Health and Social Care Partnerships Contents Ministerial Foreword for Everyone Matters Pulse Survey Report 2020 ...................... 4 Introduction ................................................................................................................. 5 Background .............................................................................................................. 5 The Pulse Survey ..................................................................................................... 6 Fieldwork .................................................................................................................. 6 Report ...................................................................................................................... 6 Staff Experience Stories .......................................................................................... 6 Response Rates Overview .......................................................................................... 7 Overall Response Rate ............................................................................................ 7 Reasons for Lower Response Rate ...................................................................... 8 Partials and Non-Completion ................................................................................ 8 Survey Methods ...................................................................................................... -

Nhs-Scotland-National-Vue-Pacs.Pdf
Patients at Centre • EPR • Single RIS • Single PACs • Local archives • National archive • Resilience of the system Health Board areas • NHS Scotland Health Boards • 1 NHS Ayrshire and Arran • 2 NHS Borders • 3 NHS Dumfries and Galloway • 4 NHS Western Isles • 5 NHS Fife • 6 NHS Forth Valley • 7 NHS Grampian • 8 NHS Greater Glasgow and Clyde • 9 NHS Highland • 10 NHS Lanarkshire • 11 NHS Lothian • 12 NHS Orkney • 13 NHS Shetland • 14 NHS Tayside Project Management of PACs in Scotland • Business Justification • Manage by exception • Focus on products • Tailor to suit the environment • Learn from experience • Manage by steps Change Control The Sudden Realisation: “The Project's Going Live“ – Develop robust Go-Live Plan – Focus on Transformational change – lasting change! • Communicate – Why? • Model behaviours • Reinforce change • Put in place robust ‘Change Control’ process – Finance – Time – Quality Scottish V11 Rollout • National Program • Largest country-wide implementation in the world • Some Sites dependent on Carestream RIS V10 • Operationally and financially complex Challenges with National Rollout • Differing needs across boards • Technology different at each board • Financial Management • Timescale management – delays have huge impact on other boards PACS v11 GG STORAGE STORAGE SPACE OO V E RR N A NN C EE ENGAGING LE A D E R SH I P Key Milestones in PACS V11 Program August 2012 -------------------------------February 2015 Project NHS GG&C NHS GG&C Project Inception Deployment Deployment Completion Starts Complete Atos Data NHS Fife