Atracurium Besilate(BAN, Rinn)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Comparative Study of Neuromuscular Blocking Effects
IndianJournalofAnesthesiaandAnalgesia OriginalResearchArticle January–February2020;7(1)(Part-I):58-63 DOI:http://dx.doi.org/10.21088/ijaa.2349.8471.7120.9 AComparativeStudyofNeuromuscularBlockingEffectsandReversibility ofCisatracuriumandVecuronium DivyaNKheskani1,HeenaSChhanwal2,BhaktiSJain3 1AssistantProfessor,2ProfessorandHead,3rdYearResident,DepartmentofAnaesthesia,GCSMedicalCollege,Hospital&Research Centre,NearChamundaBridge,Ahmedabad,Gujarat380025,India. Abstract Context: Cisatracurium is a cis isomer of parent compound atracurium, devoid of histamine release, thus possessing hemodynamic & cardiovascular stability. Aims: We compared the intubating conditions, hemodynamic stability & recovery of atracurium & vecuronium. Settings and Design: We carried out prospective, double blind randomized study after approval of ethical committee. 100 adult patients of ASA 1 &2with comparable demographicdatawereselected. Dividedin TwoGroupsC(Cisatracurium) &V(Vecuronium).Standardmonitoringwasdone&followingroutinepremedication&inductionagents, patientswereintubatedat2minsafteradministrationofcisatracurium0.15mg/kg&vecuronium0.1mg/ kgrespectively,maintainedonintermittentdoseofcisatracurium:0.03mg/kg&vecuronium:0.02mg/kg. IntubationconditionswereassessedaccordingtoTimeto25%recoveryoft1/tcfollowinginitialdoses,Time to25%recoveryoft1/tcfollowingrepeatedboluses,Timeto25%recoveryoft1/tcfollowinglastdose,Return oft4/t1ratio0.8spontaneousrecoveryatendofoperation.Statisticalanalysisused:Theresultswereevaluated byapplyingpairedt-testandp-valueusingSPSSStatisticalSoftware.Results:Intubatingconditionsat2mins -

Use of Chemical Chelators As Reversal Agents for Drug
(19) TZZ_ _ZZZ_T (11) EP 1 210 090 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: A61K 31/724 (2006.01) A61K 31/194 (2006.01) 18.06.2014 Bulletin 2014/25 A61P 39/04 (2006.01) (21) Application number: 00964006.1 (86) International application number: PCT/EP2000/007694 (22) Date of filing: 07.08.2000 (87) International publication number: WO 2001/012202 (22.02.2001 Gazette 2001/08) (54) USE OF CHEMICAL CHELATORS AS REVERSAL AGENTS FOR DRUG- INDUCED NEUROMUSCULAR BLOCK VERWENDUNG VON CHEMISCHEN CHELATOREN ZUR UMKEHRUNG VON PHARMAKOLOGISCH-INDUZIERTER NEUROMUSKULÄRER BLOCKIERUNG UTILISATION D’AGENTS CHIMIQUES CHELATANTS COMME AGENTS DE NEUTRALISATION DU BLOCAGE NEUROMUSCULAIRE PROVOQUE PAR DES MEDICAMENTS (84) Designated Contracting States: (56) References cited: AT BE CH CY DE DK ES FI FR GB GR IE IT LI LU AU-A- 3 662 895 MC NL PT SE • B DESIRE: "Inactivaton of sarin and soman by (30) Priority: 13.08.1999 EP 99306411 cyclodextrins in vitro" EXPERIENTIA, vol. 43, no. 4, 1987, pages 395-397, XP000907287 (43) Date of publication of application: • B. DESIRE: "Interaction of soman with beta- 05.06.2002 Bulletin 2002/23 cyclodextrin" FUNDAMENTAL AND APPLIED TOXICOLOGY, vol. 7, no. 4, 1986, pages 647-657, (73) Proprietor: Merck Sharp & Dohme B.V. XP000911170 2031 BN Haarlem (NL) • C. MAY: "Development of a toxin-binding agent as a treatment for tunicamycinuracil toxicity: (72) Inventors: protection against tunicamycin poisoning of • BOM, Antonius, Helena, Adolf sheep" AUSTRALIAN VETERINARY JOURNAL, Ratho, Midlothian EH28 8NY (GB) vol. 76, no. -

Comparative Study of Different Doses of Rocuronium Bromide For
MedDocs Publishers Annals of Anesthesia and Pain Medicine Open Access | Research Article Comparative study of different doses of rocuronium bromide for endotracheal intubation Nidhi V Sardhara1*; Sonal A Shah2; Dhaval P Pipaliya3; Shivansh Gupta3 1SVP hospital, 13th floor, Room no 13125, Near Ellis bridge, Ahmedabad, Gujarat-380006 2Assistant Professor, Department of anesthesia, Smt. NHL Municipal Medical College, Ahmedabad, Gujarat, India 3First year Resident, Department of anesthesia, Smt. NHL Municipal Medical College, Ahmedabad, Gujarat, India *Corresponding Author(s): Nidhi V Sardhara Abstract SVP hospital, 13th floor, Room no 13125, Near Ellis Background: Endotracheal intubation is one of such bridge, Ahmedabad, Gujarat-380006 development without which general anesthesia cannot be Tel: +91-9428-44-7878, Fax: 7926-57-8452 considered safe for any major surgery particularly head and Email: [email protected] neck, thoracic and abdominal surgeries. Ever since the ad- vent of anesthesia, anesthesiologists have been in search of an ideal muscle relaxants which can provide ideal intu- bating conditions in ultrashort duration with minimal side effects. Rocuronium bromide provides fast onset of action, Received: Mar 11, 2020 an intermediate duration of action and rapid recovery, good Accepted: Apr 24, 2020 to excellent intubating conditions at doses having minimal Published Online: Apr 30, 2020 or no haemodynamic changes. Present study is to compare the effect of different doses (0.6 mg/kg, 0.9 mg/kg, 1.2 mg/ Journal: Annals of Anesthesia and Pain Medicine kg) of Rocuronium bromide for endotracheal intubation at Publisher: MedDocs Publishers LLC 60 seconds. Online edition: http://meddocsonline.org/ Material and methods: This study was carried out by Copyright: © Sardhara NV (2020). -

Download Document
FAM001159-0001 intervals of 4-7 days to usual dose of 75-100 mg Dolmatil® (Sanofi-Synthelabo) ~ 100 rag/5 mL, thioridazine 100 rag/ per course and max. 4 injections; max. duration of daily according to response; CHILD not recom- Tablets, both scored, sulpiride 200 rag, net price ’ net price 300 mL = £7.14. Label: 2 treatment 2 weeks---if maintenance treatment mended 100-tabpack=£13.85;400mg(f/c), 100-tab ! fNote. These suspensions should not be diluted but the necessary change to an oral antipsychotic 2-3 Short-term adjunctive management of severe pack = £36¯29. Label: 2 .: :~t~a preparations may be mixed with each other to days after last injection, or to a longer acting anti- anxiety, 15-20rag daily in divided doses; max. Sulpltil® (Pharmacia) I~ ~0iovid¢ intermediate strengths psychotic depot injection given concomitantly 40 mg daily; CHILD not recommended Tablets, scored, sulpiride 200 rag. Net price 28-tab "ff~-~p, brown, thioridazine (as hydrochloride) with last injection of zuclopenthixol acetate; By deep intramuscular injection, psychoses, mania, pack = £4.29; 112-tab pack = £12.85. Label: 2 ~ff.~5"~mg/5 mL, net price 300 mL = £1.98. Label: 2 CHILD not recommended prochlorperazine mesilate 12.5-25 mg 2-3 times Sulpor® (Rosemont) IPoMI Clopi~ol Acuphase® (Lundbeck) daily; CHILD not recommended Oral solution, sugar-free, lemon- and aniseed-fla. LUOPERAZINE Injection (oily), zuclopenthixol acetate 50 mg/mL. By rectum in suppositories, psychoses, mania, the voured, sulpiride 200 mg/5 mL, net price 150 mL (’~’n~ications: see under Dose; anti-emetic (section Net price I-mL amp = £5.20; 2-mL amp = £10.03 equivalent of prochlorperazine maleate 25 mg 2- = £27.00. -

The Study Programme for the Quality Management of Essential Medicines - Good Manufacturing Practical (GMP) and Inspection
The Study Programme for the Quality Management of Essential Medicines - Good Manufacturing Practical (GMP) and Inspection - Country Reports Japan International Corporation of Welfare Services (JICWELS) Contents 1. Cambodia 1 2. Indonesia 70 3. Malaysia 91 4. Philippines 116 5. Sri Lanka 141 6. Thailand 161 The Study Programme for the Quality Management of Essential Medicines - Good Manufacturing Practical (GMP) and Inspection - Cambodia -1- KINGDOM OF CAMBODIA Nation Religion King Ministry of Health Department of Drugs and Food Country Report The Study Program on Quality Management of Essential Medicines Good Manufacturing Practice (GMP) and Inspection November 4, 2012 – November 30, 2012 Sponsored by : The Government of Japan Japan International Cooperation Agency (JICA) Department of Drugs and Food Ministry of Health, Cambodia. -2- I- COUNTRY PROFILE -3- A-Geography Cambodia is an agricultural country located in South East Asia which bordering the Gulf of Thailand, between Thailand, Vietnam, and Laos. Its approximate geographical coordinates are 13°N 105°E. Its 2,572 km border is split among Vietnam (1,228 km), Thailand (803 km) and Laos (541 km), as well as 443 km of coastline. Cambodia covers 181,035 square kilometers in the southwestern part of the Indochina, Cambodia lies completely within the tropics; its southernmost points are only slightly more than 10° above the equator. The country is bounded on the north by Thailand and by Laos, on the east and southeast by Vietnam, and on the west by the Gulf of Thailand and by Thailand. It consists of the Tonle Sap Basin and the Mekong Lowlands. To the southeast of this great basin is the Mekong Delta, which extends through Vietnam to the South China Sea. -

PMBJP Product.Pdf
Sr. Drug Generic Name of the Medicine Unit Size MRP Therapeutic Category No. Code Analgesic & Antipyretic / Muscle 1 1 Aceclofenac 100mg and Paracetamol 325 mg Tablet 10's 10's 8.00 relaxants Analgesic & Antipyretic / Muscle 2 2 Aceclofenac Tablets IP 100mg 10's 10's 4.37 relaxants Acetaminophen 325 + Tramadol Hydrochloride 37.5 film Analgesic & Antipyretic / Muscle 3 4 10's 8.00 coated Tablet 10's relaxants Analgesic & Antipyretic / Muscle 4 5 ASPIRIN Tablets IP 150 mg 14's 14's 2.70 relaxants DICLOFENAC 50 mg+ PARACETAMOL 325 mg+ Analgesic & Antipyretic / Muscle 5 6 10's 11.30 CHLORZOXAZONE 500 mg Tablets 10's relaxants Diclofenac Sodium 50mg + Serratiopeptidase 10mg Tablet Analgesic & Antipyretic / Muscle 6 8 10's 12.00 10's relaxants Analgesic & Antipyretic / Muscle 7 9 Diclofenac Sodium (SR) 100 mg Tablet 10's 10's 6.12 relaxants Analgesic & Antipyretic / Muscle 8 10 Diclofenac Sodium 25mg per ml Inj. IP 3 ml 3 ml 2.00 relaxants Analgesic & Antipyretic / Muscle 9 11 Diclofenac Sodium 50 mg Tablet 10's 10's 2.90 relaxants Analgesic & Antipyretic / Muscle 10 12 Etoricoxilb Tablets IP 120mg 10's 10's 33.00 relaxants Analgesic & Antipyretic / Muscle 11 13 Etoricoxilb Tablets IP 90mg 10's 10's 25.00 relaxants Analgesic & Antipyretic / Muscle 12 14 Ibuprofen 400 mg + Paracetamol 325 mg Tablet 10's 15's 5.50 relaxants Analgesic & Antipyretic / Muscle 13 15 Ibuprofen 200 mg film coated Tablet 10's 10's 1.80 relaxants Analgesic & Antipyretic / Muscle 14 16 Ibuprofen 400 mg film coated Tablet 10's 15's 3.50 relaxants Analgesic & Antipyretic -

Muscle Relaxants Femoral Arteriography.,,>
IN THIS ISSUE: , .:t.i ". Muscle Relaxants fI, \'.' \., Femoral ArteriographY.,,> University of Minnesota Medical Bulletin Editor ROBERT B. HOWARD, M.D. Associate Editors RAY M. AMBERG GILBERT S. CAMPBELL, M.D. ELLIS S. BENSON, MD. BYRON B. COCHRANE, M.D. E. B. BROWN, PhD. RICHARD T. SMITH, M.D. WESLEY W. SPINK, MD. University of Minnesota Medical School J. 1. MORRILL, President, University of Minnesota HAROLD S. DIEHL, MD., Dean, College of Medical SciencBs WILLIAM F. MALONEY, M.D., Assistant Dean N. 1. GAULT, JR., MD., Assistant Dean University Hospitals RAY M. AMBERG, Director Minnesota Medical Foundation WESLEY W. SPINK, M.D., President R. S. YLVISAKER, MD., Vice-President ROBERT B. HOWARD, M.D., Secretary-Treasurer Minnesota Medical Alumni Association BYRON B. COCHRANE, M.D., President VIRGIL J. P. LUNDQUIST, M.D., First Vice-President SHELDON M. LAGAARD, MD., Second Vice-President LEONARD A. BOROWICZ, M.D., Secretary JAMES C. MANKEY, M.D., Treasurer UNIVERSITY OF MINNESOTA Medical Bulletin OFFICIAL PUBLICATION OF THE UNIVERSITY OF MINNESOTA HOSPITALS, MINNE· SOTA MEDICAL FOUNDATION, AND MINNESOTA MEDICAL ALUMNI ASSOCIATION VOLUME XXVIII December 1, 1956 NUMBER 4 CONTENTS STAFF MEETING REPORTS Current Status of Muscle Relaxants BY J. Albert Jackson, MD., J. H. Matthews, M.D., J. J. Buckley, M.D., D.S.P. Weatherhead, M.D., AND F. H. Van Bergen, M.D. 114 Small Vessel Changes in Femoral Arteriography BY Alexander R. Margulis, M.D. AND T. O. Murphy, MD.__ 123 EDITORIALS .. - -. - ---__ __ _ 132 MEDICAL SCHOOL ACTIVITIES ----------- 133 POSTGRADUATE EDUCATION - 135 COMING EVENTS 136 PIIb1ished semi.monthly from October 15 to JUDe 15 at Minneapolis, Minnesota. -
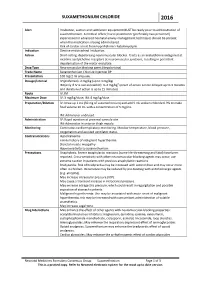
Suxamethonium Chloride 2016
SUXAMETHONIUM CHLORIDE 2016 Alert Intubation, suction and ventilation equipment MUST be ready prior to administration of suxamethonium. A medical officer/nurse practitioner (preferably two personnel) experienced in advanced neonatal airway management techniques should be present when the medication is being administered. Risk of cardiac arrest from hyperkalemic rhabdomyolysis Indication Elective endotracheal intubation. Action Short-acting, depolarising neuromuscular blocker. It acts as an acetylcholine antagonist at nicotinic acetylcholine receptors at neuromuscular junctions, resulting in persistent depolarisation of the motor end plate. Drug Type Neuromuscular blocking agent (depolarising) Trade Name Suxamethonium Chloride Injection BP Presentation 100 mg/2 ml ampoule. Dosage/Interval IV ( preferred): 2 mg/kg (up to 3 mg/kg) IM (only if IV is not accessible): 3–4 mg/kg9 (onset of action can be delayed up to 3 minutes and duration of action is up to 15 minutes) Route IV, IM Maximum Dose IV: 3 mg/kg/dose; IM: 4 mg/kg/dose Preparation/Dilution IV: Draw up 1 mL (50 mg of suxamethonium) and add 9 mL sodium chloride 0.9% to make final volume 10 mL with a concentration of 5 mg/mL. IM: Administer undiluted. Administration IV: Rapid injection at proximal cannula site. IM: Administer in anterior thigh muscle. Monitoring Continuous cardiorespiratory monitoring. Monitor temperature, blood pressure, oxygenation and assisted ventilator status. Contraindications Hyperkalaemia Family history of malignant hyperthermia Skeletal muscle myopathy Hypersensitivity to suxamethonium Precautions Anaphylaxis: Severe anaphylactic reactions (some life-threatening and fatal) have been reported. Cross-sensitivity with other neuromuscular-blocking agents may occur; use extreme caution in patients with previous anaphylactic reactions. -

Cost Analysis and Safety Comparison of Cisatracurium and Atracurium in Patients Undergoing General Anesthesia
Eur opean Rev iew for Med ical and Pharmacol ogical Sci ences 2013; 17: 447-450 Cost analysis and safety comparison of Cisatracurium and Atracurium in patients undergoing general anesthesia A. MOVAFEGH, S. AMINI*, H. SHARIFNIA, H. TORKAMANDI*, A. HAYATSHAHI*, M. JAVADI* Intensive Care Unit, and *Pharmaceutical Care Department; Dr. Shariati Hospital, Tehran University of Medical Sciences, Tehran, Iran Abstract. – BACKGROUND : Non-depolarizing Introduction neuromuscular blocking agents (NMB) differ in pharmacokinetic and pharmacodynamic parame - The introduction of neuromuscular blocking ters. An anesthesiologist according to these simi - larities and differences is able to choose the least agents in 1942 into anesthetic practice was an im - costly one if the same safety profile and same clin - portant development. Non-depolarizing NMB ical benefit achieved with the different alternatives. agents differ in the onset of action, duration of ac - AIM: The main objective of this study is to eval - tion, metabolic route, potency, adverse effects and uate the economic and adverse drug reactions cost. An anesthesiologist is able to choose NMB prevalence and differences between cisatracurium drugs according to these similarities and differ - and atracurium the two non-depolarizing NMB 1,2 drugs, which are widely used in adult patients un - ences . Atracurium and Cisatracurium are two dergoing surgery with general anesthesia in a non-depolarizing NMB agents with intermediate teaching Hospital in Iran. duration of action 1. Cisatracurium besilate is the MATERIALS AND METHODS : A cost analysis R-cis isomer of atracurium besilate and is 3-4 fold and adverse drug reactions (ADR) monitoring more potent than atracurium 3. Compared with were performed. -

Doxacurium Chloride Injection Abbott Laboratories
NUROMAX - doxacurium chloride injection Abbott Laboratories ---------- NUROMAX® (doxacurium chloride) Injection This drug should be administered only by adequately trained individuals familiar with its actions, characteristics, and hazards. NOT FOR USE IN NEONATES CONTAINS BENZYL ALCOHOL DESCRIPTION NUROMAX (doxacurium chloride) is a long-acting, nondepolarizing skeletal muscle relaxant for intravenous administration. Doxacurium chloride is [1α,2β(1'S*,2'R*)]-2,2' -[(1,4-dioxo-1,4- butanediyl)bis(oxy-3,1-propanediyl)]bis[1,2,3,4-tetrahydro-6,7,8-trimethoxy-2- methyl-1-[(3,4,5- trimethoxyphenyl)methyl]isoquinolinium] dichloride (meso form). The molecular formula is C56H78CI2N2O16 and the molecular weight is 1106.14. The compound does not partition into the 1- octanol phase of a distilled water/ 1-octanol system, i.e., the n-octanol:water partition coefficient is 0. Doxacurium chloride is a mixture of three trans, trans stereoisomers, a dl pair [(1R,1'R ,2S,2'S ) and (1S,1'S ,2R,2'R )] and a meso form (1R,1'S,2S,2'R). The meso form is illustrated below: NUROMAX Injection is a sterile, nonpyrogenic aqueous solution (pH 3.9 to 5.0) containing doxacurium chloride equivalent to 1 mg/mL doxacurium in Water for Injection. Hydrochloric acid may have been added to adjust pH. NUROMAX Injection contains 0.9% w/v benzyl alcohol. Reference ID: 2867706 CLINICAL PHARMACOLOGY NUROMAX binds competitively to cholinergic receptors on the motor end-plate to antagonize the action of acetylcholine, resulting in a block of neuromuscular transmission. This action is antagonized by acetylcholinesterase inhibitors, such as neostigmine. Pharmacodynamics NUROMAX is approximately 2.5 to 3 times more potent than pancuronium and 10 to 12 times more potent than metocurine. -

Pharmacy and Poisons (Third and Fourth Schedule Amendment) Order 2017
Q UO N T FA R U T A F E BERMUDA PHARMACY AND POISONS (THIRD AND FOURTH SCHEDULE AMENDMENT) ORDER 2017 BR 111 / 2017 The Minister responsible for health, in exercise of the power conferred by section 48A(1) of the Pharmacy and Poisons Act 1979, makes the following Order: Citation 1 This Order may be cited as the Pharmacy and Poisons (Third and Fourth Schedule Amendment) Order 2017. Repeals and replaces the Third and Fourth Schedule of the Pharmacy and Poisons Act 1979 2 The Third and Fourth Schedules to the Pharmacy and Poisons Act 1979 are repealed and replaced with— “THIRD SCHEDULE (Sections 25(6); 27(1))) DRUGS OBTAINABLE ONLY ON PRESCRIPTION EXCEPT WHERE SPECIFIED IN THE FOURTH SCHEDULE (PART I AND PART II) Note: The following annotations used in this Schedule have the following meanings: md (maximum dose) i.e. the maximum quantity of the substance contained in the amount of a medicinal product which is recommended to be taken or administered at any one time. 1 PHARMACY AND POISONS (THIRD AND FOURTH SCHEDULE AMENDMENT) ORDER 2017 mdd (maximum daily dose) i.e. the maximum quantity of the substance that is contained in the amount of a medicinal product which is recommended to be taken or administered in any period of 24 hours. mg milligram ms (maximum strength) i.e. either or, if so specified, both of the following: (a) the maximum quantity of the substance by weight or volume that is contained in the dosage unit of a medicinal product; or (b) the maximum percentage of the substance contained in a medicinal product calculated in terms of w/w, w/v, v/w, or v/v, as appropriate. -

(12) Patent Application Publication (10) Pub. No.: US 2006/0024365A1 Vaya Et Al
US 2006.0024.365A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2006/0024365A1 Vaya et al. (43) Pub. Date: Feb. 2, 2006 (54) NOVEL DOSAGE FORM (30) Foreign Application Priority Data (76) Inventors: Navin Vaya, Gujarat (IN); Rajesh Aug. 5, 2002 (IN)................................. 699/MUM/2002 Singh Karan, Gujarat (IN); Sunil Aug. 5, 2002 (IN). ... 697/MUM/2002 Sadanand, Gujarat (IN); Vinod Kumar Jan. 22, 2003 (IN)................................... 80/MUM/2003 Gupta, Gujarat (IN) Jan. 22, 2003 (IN)................................... 82/MUM/2003 Correspondence Address: Publication Classification HEDMAN & COSTIGAN P.C. (51) Int. Cl. 1185 AVENUE OF THE AMERICAS A6IK 9/22 (2006.01) NEW YORK, NY 10036 (US) (52) U.S. Cl. .............................................................. 424/468 (22) Filed: May 19, 2005 A dosage form comprising of a high dose, high Solubility active ingredient as modified release and a low dose active ingredient as immediate release where the weight ratio of Related U.S. Application Data immediate release active ingredient and modified release active ingredient is from 1:10 to 1:15000 and the weight of (63) Continuation-in-part of application No. 10/630,446, modified release active ingredient per unit is from 500 mg to filed on Jul. 29, 2003. 1500 mg, a process for preparing the dosage form. Patent Application Publication Feb. 2, 2006 Sheet 1 of 10 US 2006/0024.365A1 FIGURE 1 FIGURE 2 FIGURE 3 Patent Application Publication Feb. 2, 2006 Sheet 2 of 10 US 2006/0024.365A1 FIGURE 4 (a) 7 FIGURE 4 (b) Patent Application Publication Feb. 2, 2006 Sheet 3 of 10 US 2006/0024.365 A1 FIGURE 5 100 ov -- 60 40 20 C 2 4.