Molecular Cloning and Expression Analysis of the Endogenous Cellulase Gene Macel1 in Monochamus Alternatus
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
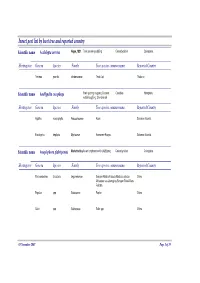
Insect Pest List by Host Tree and Reported Country
Insect pest list by host tree and reported country Scientific name Acalolepta cervina Hope, 1831 Teak canker grub|Eng Cerambycidae Coleoptera Hosting tree Genera Species Family Tree species common name Reported Country Tectona grandis Verbenaceae Teak-Jati Thailand Scientific name Amblypelta cocophaga Fruit spotting bug|eng Coconut Coreidae Hemiptera nutfall bug|Eng, Chinche del Hosting tree Genera Species Family Tree species common name Reported Country Agathis macrophylla Araucariaceae Kauri Solomon Islands Eucalyptus deglupta Myrtaceae Kamarere-Bagras Solomon Islands Scientific name Anoplophora glabripennis Motschulsky Asian longhorn beetle (ALB)|eng Cerambycidae Coleoptera Hosting tree Genera Species Family Tree species common name Reported Country Paraserianthes falcataria Leguminosae Sengon-Albizia-Falcata-Molucca albizia- China Moluccac sau-Jeungjing-Sengon-Batai-Mara- Falcata Populus spp. Salicaceae Poplar China Salix spp. Salicaceae Salix spp. China 05 November 2007 Page 1 of 35 Scientific name Aonidiella orientalis Newstead, Oriental scale|eng Diaspididae Homoptera 1894 Hosting tree Genera Species Family Tree species common name Reported Country Lovoa swynnertonii Meliaceae East African walnut Cameroon Azadirachta indica Meliaceae Melia indica-Neem Nigeria Scientific name Apethymus abdominalis Lepeletier, Tenthredinidae Hymenoptera 1823 Hosting tree Genera Species Family Tree species common name Reported Country Other Coniferous Other Coniferous Romania Scientific name Apriona germari Hope 1831 Long-horned beetle|eng Cerambycidae -

Inventory and Review of Quantitative Models for Spread of Plant Pests for Use in Pest Risk Assessment for the EU Territory1
EFSA supporting publication 2015:EN-795 EXTERNAL SCIENTIFIC REPORT Inventory and review of quantitative models for spread of plant pests for use in pest risk assessment for the EU territory1 NERC Centre for Ecology and Hydrology 2 Maclean Building, Benson Lane, Crowmarsh Gifford, Wallingford, OX10 8BB, UK ABSTRACT This report considers the prospects for increasing the use of quantitative models for plant pest spread and dispersal in EFSA Plant Health risk assessments. The agreed major aims were to provide an overview of current modelling approaches and their strengths and weaknesses for risk assessment, and to develop and test a system for risk assessors to select appropriate models for application. First, we conducted an extensive literature review, based on protocols developed for systematic reviews. The review located 468 models for plant pest spread and dispersal and these were entered into a searchable and secure Electronic Model Inventory database. A cluster analysis on how these models were formulated allowed us to identify eight distinct major modelling strategies that were differentiated by the types of pests they were used for and the ways in which they were parameterised and analysed. These strategies varied in their strengths and weaknesses, meaning that no single approach was the most useful for all elements of risk assessment. Therefore we developed a Decision Support Scheme (DSS) to guide model selection. The DSS identifies the most appropriate strategies by weighing up the goals of risk assessment and constraints imposed by lack of data or expertise. Searching and filtering the Electronic Model Inventory then allows the assessor to locate specific models within those strategies that can be applied. -

Forestry Department Food and Agriculture Organization of the United Nations
Forestry Department Food and Agriculture Organization of the United Nations Forest Health & Biosecurity Working Papers OVERVIEW OF FOREST PESTS THAILAND January 2007 Forest Resources Development Service Working Paper FBS/32E Forest Management Division FAO, Rome, Italy Forestry Department Overview of forest pests – Thailand DISCLAIMER The aim of this document is to give an overview of the forest pest1 situation in Thailand. It is not intended to be a comprehensive review. The designations employed and the presentation of material in this publication do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. © FAO 2007 1 Pest: Any species, strain or biotype of plant, animal or pathogenic agent injurious to plants or plant products (FAO, 2004). ii Overview of forest pests – Thailand TABLE OF CONTENTS Introduction..................................................................................................................... 1 Forest pests...................................................................................................................... 1 Naturally regenerating forests..................................................................................... 1 Insects ..................................................................................................................... 1 Diseases.................................................................................................................. -

Ramesh Chander Bhagat.Pdf
Int. J. Curr. Res. Biosci. Plant Biol. 2016, 3(12): 115-123 International Journal of Current Research in Biosciences and Plant Biology ISSN: 2349-8080 (Online) ● Volume 3 ● Number 12 (December-2016) Journal homepage: www.ijcrbp.com Original Research Article doi: http://dx.doi.org/10.20546/ijcrbp.2016.312.014 An Update on Checklist and Biodiversity of Coleopteran-fauna (Insecta) of Forestry and Mulberry Importance in Jammu and Kashmir State (India) Ramesh Chander Bhagat* P.O. Box No. 1250, G.P.O., Residency Road, Srinagar, Kashmir-190 001, J & K, India *Corresponding author. A b s t r a c t Article Info The present paper deals with a total of 64 species of beetles and weevils (Coleoptera), Accepted: 29 November 2016 belonging to 52 genera, under 14 families, associated with diverse species of forest and Available Online: 06 December 2016 mulberry plantations, occurring in vast areas and localities of Jammu and Kashmir State. The Coleopteran species of forestry and mulberry importance accounts for 73.43 K e y w o r d s % and 35.93 % respectively. The Coleopteran-fauna (47 spp.), spread over 12 families, is found to be infesting forest trees,viz. Ash, Benne, Birch, Conifers, Elms, Biodiversity Ivy, Maple, Oak, Parrotia, Plane tree, Poplars, Robinia, Salix, and Yew. Of these trees, Checklist Pines showed highest number of Coleopteran species i.e. 18, under 6 families, followed Coleopteran-fauna by Poplars, with 15 spp. (4 families) and Cedars, having 14 spp. (4 families) The Forest trees Mulberry plantations Mulberry plantations (Morus spp.) both endemic as well as exotic, have been observed to be infesting 23 spp. -

PRA on Apriona Species
EUROPEAN AND MEDITERRANEAN PLANT PROTECTION ORGANIZATION ORGANISATION EUROPEENNE ET MEDITERRANEENNE POUR LA PROTECTION DES PLANTES 16-22171 (13-18692) Only the yellow note is new compared to document 13-18692 Pest Risk Analysis for Apriona germari, A. japonica, A. cinerea Note: This PRA started 2011; as a result, three species of Apriona were added to the EPPO A1 List: Apriona germari, A. japonica and A. cinerea. However recent taxonomic changes have occurred with significant consequences on their geographical distributions. A. rugicollis is no longer considered as a synonym of A. germari but as a distinct species. A. japonica, which was previously considered to be a distinct species, has been synonymized with A. rugicollis. Finally, A. cinerea remains a separate species. Most of the interceptions reported in the EU as A. germari are in fact A. rugicollis. The outcomes of the PRA for these pests do not change. However A. germari has a more limited and a more tropical distribution than originally assessed, but it is considered that it could establish in Southern EPPO countries. The Panel on Phytosanitary Measures agreed with the addition of Apriona rugicollis to the A1 list. September 2013 EPPO 21 Boulevard Richard Lenoir 75011 Paris www.eppo.int [email protected] This risk assessment follows the EPPO Standard PM PM 5/3(5) Decision-support scheme for quarantine pests (available at http://archives.eppo.int/EPPOStandards/pra.htm) and uses the terminology defined in ISPM 5 Glossary of Phytosanitary Terms (available at https://www.ippc.int/index.php). This document was first elaborated by an Expert Working Group and then reviewed by the Panel on Phytosanitary Measures and if relevant other EPPO bodies. -

A First Report of the Bamboo Weevil Cyrtotrachelus Sp. As a Serious Pest of Managa Bamboo Dendrocalamus Stocksii (Munro) in Ratnagiri District, Maharashtra, India
J. Bamboo and Rattan, Vol. 16, No. 1, pp. 23-32 (2017) c KFRI 2017 A first report of the Bamboo weevil Cyrtotrachelus sp. as a serious pest of Managa bamboo Dendrocalamus stocksii (Munro) in Ratnagiri district, Maharashtra, India Milind Digambar Patil University of Mumbai, M. G. Road, Fort, Mumbai 400032, India. Abstract: Dendrocalamus stocksii is a commercially important bamboo species in Peninsular India. The Bamboo weevil Cyrtotrachelus sp. (Coleoptera: Curculionidae) is reported for the first time as a shoot borer of tender shoots of D. stocksii at Dapoli, Maharashtra, India. A stagnant rancid odour in the plantation first indicated heavy infestation of the pest. As much as 62% of the newly emerging shoots showed infestation. Around 94% of the incidences were recorded within 1.5m above ground surface. Tunneling by the grubs resulted in terminal shoot damage and led to the formation of epicormics. Observations on the biology, infestation status and economic significance of this pest are presented. Keywords: Bamboo pests, earthen puparia, entomophilic nematodes, insect pheromones, Western Ghats INTRODUCTION Dendrocalamus stocksii (Munro) M. Kumar, Remesh and Unnikrishnan, 2004 is a medium sized, sympodial bamboo species found in the Central Western Ghats. It is distributed from northern Kerala, Karnataka and Goa up to the Konkan coasts of Maharashtra (Kumar et al., 2004). It has wide physiographical adaptability and comes up well in tropical humid, sub humid and semi-arid conditions (Viswanath et al., 2012). D. stocksii is traditionally being planted in the home gardens, farm bunds, farm borders and for bio-fencing (Rane et al., 2016). It is the third most preferred bamboo species in agriculture sector in peninsular India (Rao et al., 2008). -

Drug Discovery Insights from Medicinal Beetles in Traditional Chinese Medicine
Review Biomol Ther 29(2), 105-126 (2021) Drug Discovery Insights from Medicinal Beetles in Traditional Chinese Medicine Stephen T. Deyrup1,*, Natalie C. Stagnitti1, Mackenzie J. Perpetua1 and Siu Wah Wong-Deyrup2 1Department of Chemistry and Biochemistry, Siena College, Loudonville, NY 12309, 2The RNA Institute and Department of Biological Sciences, University at Albany, State University of New York, Albany, NY 12222, USA Abstract Traditional Chinese medicine (TCM) was the primary source of medical treatment for the people inhabiting East Asia for thousands of years. These ancient practices have incorporated a wide variety of materia medica including plants, animals and minerals. As modern sciences, including natural products chemistry, emerged, there became increasing efforts to explore the chemistry of this materia medica to find molecules responsible for their traditional use. Insects, including beetles have played an important role in TCM. In our survey of texts and review articles on TCM materia medica, we found 48 species of beetles from 34 genera in 14 different families that are used in TCM. This review covers the chemistry known from the beetles used in TCM, or in cases where a species used in these practices has not been chemically studied, we discuss the chemistry of closely related beetles. We also found several documented uses of beetles in Traditional Korean Medicine (TKM), and included them where appropriate. There are 129 chemical constituents of beetles discussed. Key Words: Beetle, Traditional Chinese Medicine, Traditional Korean Medicine, Coleoptera, Chemical defense, Secondary metabolites INTRODUCTION toms. There are several guiding philosophies and treatment modalities including acupuncture, moxibustion, and qi gong Traditional Chinese Medicine (TCM) is widely used both in- (Liu and Liu, 2009; Fung and Linn, 2015; National Center for side China and beyond its borders. -

Larval Peritrophic Matrix Yu-Bo Lin1,2†, Jing-Jing Rong1,2†, Xun-Fan Wei3, Zhuo-Xiao Sui3, Jinhua Xiao3 and Da-Wei Huang3*
Lin et al. Proteome Science (2021) 19:7 https://doi.org/10.1186/s12953-021-00175-x RESEARCH Open Access Proteomics and ultrastructural analysis of Hermetia illucens (Diptera: Stratiomyidae) larval peritrophic matrix Yu-Bo Lin1,2†, Jing-Jing Rong1,2†, Xun-Fan Wei3, Zhuo-Xiao Sui3, Jinhua Xiao3 and Da-Wei Huang3* Abstract Background: The black soldier fly (Hermetia illucens) has significant economic potential. The larvae can be used in financially viable waste management systems, as they are voracious feeders able to efficiently convert low-quality waste into valuable biomass. However, most studies on H. illucens in recent decades have focused on optimizing their breeding and bioconversion conditions, while information on their biology is limited. Methods: About 200 fifth instar well-fed larvae were sacrificed in this work. The liquid chromatography-tandem mass spectrometry and scanning electron microscopy were employed in this study to perform a proteomic and ultrastructural analysis of the peritrophic matrix (PM) of H. illucens larvae. Results: A total of 565 proteins were identified in the PM samples of H. illucen, of which 177 proteins were predicted to contain signal peptides, bioinformatics analysis and manual curation determined 88 proteins may be associated with the PM, with functions in digestion, immunity, PM modulation, and others. The ultrastructure of the H. illucens larval PM observed by scanning electron microscopy shows a unique diamond-shaped chitin grid texture. Conclusions: It is the first and most comprehensive proteomics research about the PM of H. illucens larvae to date. All the proteins identified in this work has been discussed in details, except several unnamed or uncharacterized proteins, which should not be ignored and need further study. -

New Records of Longhorn Beetles (Cerambycidae: Coleoptera) From
The Journal of Zoology Studies 2016; 3(1): 19-26 The Journal of Zoology Studies ISSN 2348-5914 New records of longhorn beetles (Cerambycidae: Coleoptera) JOZS 2016; 3(1): 19-26 from Manipur State India with Checklist JOZS © 2016 Received: 11-02-2016 Author: Bulganin Mitra, Priyanka Das, Kaushik Mallick, Udipta Chakraborti and Amitava Majumder Accepted: 22-03-2016 Abstract Bulganin Mitra, Present communication reports 50 species of 40 genera under 24 tribes belonging to 5 Scientist – C Zoological Survey of India, Subfamilies of the family Cerambycidae from the state of Manipur. Among them, Dorysthenes Kolkata – 700053. India (Lophosternus) huegelii (Redtenbacher, 1848) and Olenecamptus bilobus (Fabricius, 1801) are Priyanka Das recorded for the first time from Manipur state. Moreover, 20 % of the total cerambycid fauna is Junior Research Fellow, Zoological Survey of India, restricted/endemic to the state. Kolkata – 700053. India Keywords: India, Manipur, Cerambycidae, New record Kaushik Mallick Post Graduate Department of 1. Introduction Zoology, Ashutosh College, Manipur is one of the seven states of Northeast India, lies at a latitude of 23°83′ N to 25°68′ N Kolkata – 700026. and a longitude of 93°03′ E to 94°78′ E. The total area covered by the state is 22,347 km². The India natural vegetation occupies an area of about 14,365 km² which is nearly 64% of the total Udipta Chakraborti, geographical area of the state and which attracts a large number of forest pests. Junior Research Fellow, Zoological Survey of India, Kolkata – 700053. The members of the family Cerambycidae are commonly known as longhorn beetles or round- India headed borer beetles, is one of the notorious groups of insect pest due to their diurnal and Amitava Majumder nocturnal activities. -

Variation in Inspection Efficacy by Member States of Wood Packaging Material Entering the European Union
Journal of Economic Entomology, 111(2), 2018, 707–715 doi: 10.1093/jee/tox357 Advance Access Publication Date: 20 January 2018 Forest Entomology Research Article Variation in Inspection Efficacy by Member States of Wood Packaging Material Entering the European Union Dominic Eyre,1,5 Roy Macarthur,2 Robert A. Haack,3 Yi Lu,2 and Hannes Krehan4 1Defra, Sand Hutton, York YO41 1LZ, UK, 2Fera Science Ltd., Sand Hutton, York YO41 1LZ, UK, 3USDA Forest Service, Northern Research Station, 3101 Technology Boulevard, Suite F, Lansing, MI 48910 (Emeritus), 4Bundesamt für Wald, Seckendorff- Gudent-Weg 8, 1131 Wien, Austria, and 5Corresponding author, e-mail: [email protected] Subject Editor: Lisa Neven Received 31 August 2017; Editorial decision 21 November 2017 Abstract The use of wood packaging materials (WPMs) in international trade is recognized as a pathway for the movement of invasive pests and as the origin of most introductions of Asian longhorned beetle, Anoplophora glabripennis (Motschulsky) (Coleoptera: Cerambycidae) in Europe and North America. Following several pest interceptions on WPM associated with stone imports from China, the European Union (EU) agreed to survey certain categories of imports based on the EU Combined Nomenclature Codes for imports, which are based on the international Harmonized System. Between April 2013 and March 2015, 72,263 relevant consignments were received from China in the EU and 26,008 were inspected. Harmful organisms were detected in 0.9% of the consignments, and 1.1% of the imports did not have markings compliant with the international standard for treating WPM, ISPM 15. There were significant differences between the detection rates of harmful organisms among EU member states. -

Identification of Novel Catalytic Features of Endo-Β-1,4-Glucanase Produced by Mulberry Longicorn Beetle Apriona Germari
Sami et al. / J Zhejiang Univ Sci B 2007 8(10):765-770 765 Journal of Zhejiang University SCIENCE B ISSN 1673-1581 (Print); ISSN 1862-1783 (Online) www.zju.edu.cn/jzus; www.springerlink.com E-mail: [email protected] Identification of novel catalytic features of endo-β-1,4-glucanase produced by mulberry longicorn beetle Apriona germari SAMI Amtul Jamil†, HAIDER Mohammed Kamran (Institute of Biochemistry and Biotechnology, University of the Punjab, Lahore 54594, Pakistan) †E-mail: [email protected]; [email protected] Received July 30, 2007; revision accepted Sept. 7, 2007 Abstract: Mulberry longicorn beetle, Apriona germari, has been reported to produce two endo-β-1,4-glucanases or AgEGases (accession Nos. Q6SS52 and Q5XQD1). AgEGase sequence contains catalytic motif (amino acid residues 37~48), which is the characteristic of family Glycohydrolase 45 and is identified as the substrate binding site. The application of bioinformatics ap- proaches includes sequence analysis, structural modeling and inhibitor docking to relate the structure and function of AgEGases. We have dissected the sequence and structure of AgEGase catalytic motif and compared it with crystal structure of Humicola insolens endoglucanases V. The results show an involvement of sulfur containing amino acid residues in the active site of the enzyme. Cys residues and position of disulfide bonds are highly conserved between the two structures of endoglucanases of A. germari. Surface calculation of AgEGase structure in the absence of Cys residues reveals greater accessibility of the catalytic site to the substrate involving Asp42, a highly conserved residue. For the inhibition study, tannin-based structure was docked into the catalytic site of AgEGase using ArgusLab 4.0 and it resulted in a stable complex formation. -

The Major Arthropod Pests and Weeds of Agriculture in Southeast Asia
The Major Arthropod Pests and Weeds of Agriculture in Southeast Asia: Distribution, Importance and Origin D.F. Waterhouse (ACIAR Consultant in Plant Protection) ACIAR (Australian Centre for International Agricultural Research) Canberra AUSTRALIA The Australian Centre for International Agricultural Research (ACIAR) was established in June 1982 by an Act of the Australian Parliament. Its mandate is to help identify agricultural problems in developing countries and to commission collaborative research between Australian and developing country researchers in fields where Australia has a special research competence. Where trade names are used this constitutes neither endorsement of nor discrimination against any product by the Centre. ACIAR MO'lOGRAPH SERIES This peer-reviewed series contains the results of original research supported by ACIAR, or deemed relevant to ACIAR's research objectives. The series is distributed internationally, with an emphasis on the Third World. © Australian Centre for 1I1lernational Agricultural Resl GPO Box 1571, Canberra, ACT, 2601 Waterhouse, D.F. 1993. The Major Arthropod Pests an Importance and Origin. Monograph No. 21, vi + 141pI- ISBN 1 86320077 0 Typeset by: Ms A. Ankers Publication Services Unit CSIRO Division of Entomology Canberra ACT Printed by Brown Prior Anderson, 5 Evans Street, Burwood, Victoria 3125 ii Contents Foreword v 1. Abstract 2. Introduction 3 3. Contributors 5 4. Results 9 Tables 1. Major arthropod pests in Southeast Asia 10 2. The distribution and importance of major arthropod pests in Southeast Asia 27 3. The distribution and importance of the most important arthropod pests in Southeast Asia 40 4. Aggregated ratings for the most important arthropod pests 45 5. Origin of the arthropod pests scoring 5 + (or more) or, at least +++ in one country or ++ in two countries 49 6.