Larval Peritrophic Matrix Yu-Bo Lin1,2†, Jing-Jing Rong1,2†, Xun-Fan Wei3, Zhuo-Xiao Sui3, Jinhua Xiao3 and Da-Wei Huang3*
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Chymotrypsin from the Digestive Tract of California Spiny Lobster, Panulirus Interruptus: Purification and Biochemical Characterization
A chymotrypsin from the Digestive Tract of California Spiny Lobster, Panulirus interruptus: Purification and Biochemical Characterization Betsaida Bibo-Verdugo, Liliana Rojo- Arreola, Maria A. Navarrete-del-Toro & Fernando García-Carreño Marine Biotechnology An International Journal Focusing on Marine Genomics, Molecular Biology and Biotechnology ISSN 1436-2228 Volume 17 Number 4 Mar Biotechnol (2015) 17:416-427 DOI 10.1007/s10126-015-9626-z 1 23 Your article is protected by copyright and all rights are held exclusively by Springer Science +Business Media New York. This e-offprint is for personal use only and shall not be self- archived in electronic repositories. If you wish to self-archive your article, please use the accepted manuscript version for posting on your own website. You may further deposit the accepted manuscript version in any repository, provided it is only made publicly available 12 months after official publication or later and provided acknowledgement is given to the original source of publication and a link is inserted to the published article on Springer's website. The link must be accompanied by the following text: "The final publication is available at link.springer.com”. 1 23 Author's personal copy Mar Biotechnol (2015) 17:416–427 DOI 10.1007/s10126-015-9626-z ORIGINAL ARTICLE A chymotrypsin from the Digestive Tract of California Spiny Lobster, Panulirus interruptus: Purification and Biochemical Characterization Betsaida Bibo-Verdugo1 & Liliana Rojo-Arreola1 & Maria A. Navarrete-del-Toro1 & Fernando García-Carreño1 Received: 13 August 2014 /Accepted: 31 January 2015 /Published online: 16 April 2015 # Springer Science+Business Media New York 2015 Abstract A chymotrypsin was purified from the gastric juice Introduction of California spiny lobster (Panulirus interrutpus), using pre- parative electrophoresis and affinity chromatography on aga- Proteolytic enzymes from the digestive system of crustacean rose-p-aminobenzamidine. -

Lesser Mealworm, Litter Beetle, Alphitobius Diaperinus (Panzer) (Insecta: Coleoptera: Tenebrionidae)1 James C
EENY-367 Lesser Mealworm, Litter Beetle, Alphitobius diaperinus (Panzer) (Insecta: Coleoptera: Tenebrionidae)1 James C. Dunford and Phillip E. Kaufman2 Introduction encountered in stored products (Green 1980). The other known species in the United States, A. laevigatus (Fabricius) The lesser mealworm, Alphitobius diaperinus (Panzer), is or black fungus beetle, is less commonly encountered and a cosmopolitan general stored products pest of particular may also vector pathogens and parasites and occasionally importance as a vector and competent reservoir of several cause damage to poultry housing. poultry pathogens and parasites. It can also cause damage to poultry housing and is suspected to be a health risk to humans in close contact with larvae and adults. Adults can become a nuisance when they move en masse toward artificial lights generated by residences near fields where beetle-infested manure has been spread (Axtell 1999). Alphitobius diaperinus inhabits poultry droppings and litter and is considered a significant pest in the poultry industry. Numerous studies have been conducted on lesser meal- worm biology, physiology, and management. Lambkin (2001) conducted a thorough review of relevant scientific literature in reference to A. diaperinus and provides a good understanding of the biology, ecology and bionomics of the pest. Bruvo et al. (1995) conducted molecular work to determine satellite DNA variants on the chromosomes of A. diaperinus. Alphitobius diaperinus is a member of the tenebrionid tribe Alphitobiini (Doyen 1989), which comprises four genera worldwide (Aalbu et al. 2002). Two genera occur Figure 1. Adult male lesser mealworm, Alphitobius diaperinus (Panzer). in the United States, of which there are two species in the This specimen taken from Henderson County, North Carolina. -

Coleoptera: Carabidae: Brachininae) from Northern Australia Martin Baehr
RECORDS OF THE WESTERN AUSTRALIAN MUSEUM 27 062–067 (2012) A new species of the genus Pheropsophus (Coleoptera: Carabidae: Brachininae) from northern Australia Martin Baehr Zoologische Staatssammlung, Münchhausenstr. 21, D–81247 München, Germany. Email: [email protected] ABSTRACT – A new species of the brachinine genus Pheropsophus Solier is described from far northern and north-western Australia: P. windjanae sp. nov. The new species which previously was not discriminated from P. verticalis (Dejean) is distinguished from that species by short and wide, posteriad considerably widened elytra, more cordiform pronotum, almost completely black base of the head, absence of a distinct yellow apical elytral margin, narrower aedeagus the apex of which is slightly sinuate on the left side, and the narrow, parallel-sided sulcus on the lower surface of the aedeagus. A revised key to the Australian species of the genus Pheropsophus is provided. KEYWORDS: taxonomy, morphology, beetle INTRODUCTION shorter and wider, posteriad considerably widened Giachino (2003) published a revision of the Australian elytra, have a more cordiform prothorax, and differ in species of the brachinine genus Pheropsophus Solier, the colouration of the head and elytra. Dissection of 1833 and examined and identifi ed in this paper a large the male genitalia of several specimens from a number number of specimens that I had collected during several of northern localities which possess the mentioned collecting trips in various parts of Australia. Apart from character states, and of a number of specimens of a few species described as new in Giachino’s paper, P. verticalis from various parts of Australia, now the bulk of the material was identifi ed as P. -

Arachnida, Solifugae) with Special Focus on Functional Analyses and Phylogenetic Interpretations
HISTOLOGY AND ULTRASTRUCTURE OF SOLIFUGES Comparative studies of organ systems of solifuges (Arachnida, Solifugae) with special focus on functional analyses and phylogenetic interpretations HISTOLOGIE UND ULTRASTRUKTUR DER SOLIFUGEN Vergleichende Studien an Organsystemen der Solifugen (Arachnida, Solifugae) mit Schwerpunkt auf funktionellen Analysen und phylogenetischen Interpretationen I N A U G U R A L D I S S E R T A T I O N zur Erlangung des akademischen Grades doctor rerum naturalium (Dr. rer. nat.) an der Mathematisch-Naturwissenschaftlichen Fakultät der Ernst-Moritz-Arndt-Universität Greifswald vorgelegt von Anja Elisabeth Klann geboren am 28.November 1976 in Bremen Greifswald, den 04.06.2009 Dekan ........................................................................................................Prof. Dr. Klaus Fesser Prof. Dr. Dr. h.c. Gerd Alberti Erster Gutachter .......................................................................................... Zweiter Gutachter ........................................................................................Prof. Dr. Romano Dallai Tag der Promotion ........................................................................................15.09.2009 Content Summary ..........................................................................................1 Zusammenfassung ..........................................................................5 Acknowledgments ..........................................................................9 1. Introduction ............................................................................ -

Genetic Evidence for a Protective Role of the Peritrophic Matrix Against Intestinal Bacterial Infection in Drosophila Melanogaster
Genetic evidence for a protective role of the peritrophic matrix against intestinal bacterial infection in Drosophila melanogaster Takayuki Kuraishi1, Olivier Binggeli, Onya Opota, Nicolas Buchon, and Bruno Lemaitre1 Global Health Institute, École Polytechnique Fédérale de Lausanne, 1015 Lausanne, Switzerland Edited by Alexander S. Raikhel, University of California, Riverside, CA, and approved August 17, 2011 (received for review April 14, 2011) The peritrophic matrix (PM) forms a layer composed of chitin and of the cytokine Upd3 from damaged enterocytes, which then glycoproteins that lines the insect intestinal lumen. This physical activates the JAK/STAT pathway in intestinal stem cells to pro- barrier plays a role analogous to that of mucous secretions of the mote both their division and differentiation, establishing a ho- vertebrate digestive tract and is thought to protect the midgut meostatic regulatory loop (9, 10). Interestingly, both Imd pathway epithelium from abrasive food particles and microbes. Almost activity and epithelium renewal are also stimulated at a basal level nothing is known about PM functions in Drosophila, and its function by the indigenous gut microbiota (10). as an immune barrier has never been addressed by a genetic ap- The peritrophic matrix (PM) forms a layer composed of chitin proach. Here we show that the Drosocrystallin (Dcy) protein, a pu- and glycoproteins that lines the insect midgut lumen (11, 12). tative component of the eye lens of Drosophila, contributes to adult Although structurally different, it plays a role analogous to that of PM formation. A loss-of-function mutation in the dcy gene results mucous secretions of the vertebrate digestive tract and is thought in a reduction of PM width and an increase of its permeability. -

Vsgovercomesanearlybarriertos
COMMENTARY VSG overcomes an early barrier to survival of African trypanosomes in tsetse flies COMMENTARY Shaden Kamhawia,1 and Iliano V. Coutinho-Abreua The widespread expansion of vector-borne diseases is susceptible to infection compared with adults that a testament to their success. According to the World produce a mature PM (13). Importantly, the mecha- Health Organization, over half the global population is nism by which trypanosomes traverse the PM to en- at risk for contracting a vector-borne disease, and over able gut colonization in susceptible tsetse had been a million deaths are annually attributed to diseases unknown until now. In PNAS, Aksoy et al. (14) share transmitted by insects (1). An absence of preventative their discovery of how trypanosomes disrupt the vaccines combined with the rising resistance to insec- PM of the tsetse fly and implicate the variant sur- ticides has led to a surge in efforts to develop alter- face glycoprotein (VSG) of the blood stream form nate approaches toward vector control. One such (BSF)—famed for its critical part in escaping the im- approach is the interruption of pathogen transmission. mune system of the mammalian host (15)—in its Understanding the molecular basis of parasite–vector disruption, revealing a dual role for VSG in the life interactions can identify critical steps in pathogen de- cycle of trypanosomes. velopment to be targeted for disruption. After millions Infected blood ingested by a tsetse fly contains a of years adapting to their vectors, pathogens have large number of slender and a few stumpy BSF try- evolved complex and innovative survival strategies panosomes, both expressing a similar coat of VSG aimed at overcoming host defenses. -

Serine Proteases with Altered Sensitivity to Activity-Modulating
(19) & (11) EP 2 045 321 A2 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 08.04.2009 Bulletin 2009/15 C12N 9/00 (2006.01) C12N 15/00 (2006.01) C12Q 1/37 (2006.01) (21) Application number: 09150549.5 (22) Date of filing: 26.05.2006 (84) Designated Contracting States: • Haupts, Ulrich AT BE BG CH CY CZ DE DK EE ES FI FR GB GR 51519 Odenthal (DE) HU IE IS IT LI LT LU LV MC NL PL PT RO SE SI • Coco, Wayne SK TR 50737 Köln (DE) •Tebbe, Jan (30) Priority: 27.05.2005 EP 05104543 50733 Köln (DE) • Votsmeier, Christian (62) Document number(s) of the earlier application(s) in 50259 Pulheim (DE) accordance with Art. 76 EPC: • Scheidig, Andreas 06763303.2 / 1 883 696 50823 Köln (DE) (71) Applicant: Direvo Biotech AG (74) Representative: von Kreisler Selting Werner 50829 Köln (DE) Patentanwälte P.O. Box 10 22 41 (72) Inventors: 50462 Köln (DE) • Koltermann, André 82057 Icking (DE) Remarks: • Kettling, Ulrich This application was filed on 14-01-2009 as a 81477 München (DE) divisional application to the application mentioned under INID code 62. (54) Serine proteases with altered sensitivity to activity-modulating substances (57) The present invention provides variants of ser- screening of the library in the presence of one or several ine proteases of the S1 class with altered sensitivity to activity-modulating substances, selection of variants with one or more activity-modulating substances. A method altered sensitivity to one or several activity-modulating for the generation of such proteases is disclosed, com- substances and isolation of those polynucleotide se- prising the provision of a protease library encoding poly- quences that encode for the selected variants. -

ICAR–NBAIR Annual Report 2019.Pdf
Annual Report 2019 ICAR–NATIONAL BUREAU OF AGRICULTURAL INSECT RESOURCES Bengaluru 560 024, India Published by The Director ICAR–National Bureau of Agricultural Insect Resources P.O. Box 2491, H.A. Farm Post, Hebbal, Bengaluru 560 024, India Phone: +91 80 2341 4220; 2351 1998; 2341 7930 Fax: +91 80 2341 1961 E-mail: [email protected] Website: www.nbair.res.in ISO 9001:2008 Certified (No. 6885/A/0001/NB/EN) Compiled and edited by Prakya Sreerama Kumar Amala Udayakumar Mahendiran, G. Salini, S. David, K.J. Bakthavatsalam, N. Chandish R. Ballal Cover and layout designed by Prakya Sreerama Kumar May 2020 Disclaimer ICAR–NBAIR neither endorses nor discriminates against any product referred to by a trade name in this report. Citation ICAR–NBAIR. 2020. Annual Report 2019. ICAR–National Bureau of Agricultural Insect Resources, Bengaluru, India, vi + 105 pp. Printed at CNU Graphic Printers 35/1, South End Road Malleswaram, Bengaluru 560 020 Mobile: 9880 888 399 E-mail: [email protected] CONTENTS Preface ..................................................................................................................................... v 1. Executive Summary................................................................................................................ 1 2. Introduction ............................................................................................................................ 6 3. Research Achievements .......................................................................................................11 -

(L. 1758): on the Origin of Fatty Acids in Prepupae B
www.nature.com/scientificreports OPEN About lipid metabolism in Hermetia illucens (L. 1758): on the origin of fatty acids in prepupae B. Hoc1, M. Genva2, M.‑L. Fauconnier2, G. Lognay1, F. Francis1 & R. Caparros Megido1* Although increasingly targeted in animal nutrition, black soldier fy larvae or prepupae (BSF, Hermetia illucens L. 1758) require the characterization and modulation of their fatty acid profle to become fully integrated within the feed sector. This improvement will only be possible by the understanding of underlaying biochemical pathways of fatty acid synthesis in BSF. In this study, we hypothesized a labelling of de novo synthesized fatty acids in BSF by the incorporation of deuterated water (D2O) in their feed. Three batches of ffty larvae were reared on two diets with diferent polyunsaturated fatty acid profles moistened with 40% of H2O or D2O: chicken feed or 40% of chicken feed and 60% of fax cake. Although the occurrence of D2O in insect feed increased the larval development time and decreased prepupal weight, it was possible to track the biosynthesis of fatty acids through deuterium labelling. Some fatty acids (decanoic, lauric or myristic acid) were exclusively present in their deuterated form while others (palmitic, palmitoleic or oleic acid) were found in two forms (deuterated or not) indicating that BSF can partially produce these fatty acids via biosynthesis pathways and not only by bioaccumulation from the diet. These results suggest the importance of carbohydrates as a source of acetyl‑CoA in the constitution of the BSF fatty acid profle but also the potential importance of specifc enzymes (e.g. -
![Ichneumonid Wasps (Hymenoptera, Ichneumonidae) in the to Scale Caterpillar (Lepidoptera) [1]](https://docslib.b-cdn.net/cover/0863/ichneumonid-wasps-hymenoptera-ichneumonidae-in-the-to-scale-caterpillar-lepidoptera-1-720863.webp)
Ichneumonid Wasps (Hymenoptera, Ichneumonidae) in the to Scale Caterpillar (Lepidoptera) [1]
Central JSM Anatomy & Physiology Bringing Excellence in Open Access Research Article *Corresponding author Bui Tuan Viet, Institute of Ecology an Biological Resources, Vietnam Acedemy of Science and Ichneumonid Wasps Technology, 18 Hoang Quoc Viet, Cau Giay, Hanoi, Vietnam, Email: (Hymenoptera, Ichneumonidae) Submitted: 11 November 2016 Accepted: 21 February 2017 Published: 23 February 2017 Parasitizee a Pupae of the Rice Copyright © 2017 Viet Insect Pests (Lepidoptera) in OPEN ACCESS Keywords the Hanoi Area • Hymenoptera • Ichneumonidae Bui Tuan Viet* • Lepidoptera Vietnam Academy of Science and Technology, Vietnam Abstract During the years 1980-1989,The surveys of pupa of the rice insect pests (Lepidoptera) in the rice field crops from the Hanoi area identified showed that 12 species of the rice insect pests, which were separated into three different groups: I- Group (Stem bore) including Scirpophaga incertulas, Chilo suppressalis, Sesamia inferens; II-Group (Leaf-folder) including Parnara guttata, Parnara mathias, Cnaphalocrocis medinalis, Brachmia sp, Naranga aenescens; III-Group (Bite ears) including Mythimna separata, Mythimna loryei, Mythimna venalba, Spodoptera litura . From these organisms, which 15 of parasitoid species were found, those species belonging to 5 families in of the order Hymenoptera (Ichneumonidae, Chalcididae, Eulophidae, Elasmidae, Pteromalidae). Nine of these, in which there were 9 of were ichneumonid wasp species: Xanthopimpla flavolineata, Goryphus basilaris, Xanthopimpla punctata, Itoplectis naranyae, Coccygomimus nipponicus, Coccygomimus aethiops, Phaeogenes sp., Atanyjoppa akonis, Triptognatus sp. We discuss the general biology, habitat preferences, and host association of the knowledge of three of these parasitoids, (Xanthopimpla flavolineata, Phaeogenes sp., and Goryphus basilaris). Including general biology, habitat preferences and host association were indicated and discussed. -

A Genus-Level Supertree of Adephaga (Coleoptera) Rolf G
ARTICLE IN PRESS Organisms, Diversity & Evolution 7 (2008) 255–269 www.elsevier.de/ode A genus-level supertree of Adephaga (Coleoptera) Rolf G. Beutela,Ã, Ignacio Riberab, Olaf R.P. Bininda-Emondsa aInstitut fu¨r Spezielle Zoologie und Evolutionsbiologie, FSU Jena, Germany bMuseo Nacional de Ciencias Naturales, Madrid, Spain Received 14 October 2005; accepted 17 May 2006 Abstract A supertree for Adephaga was reconstructed based on 43 independent source trees – including cladograms based on Hennigian and numerical cladistic analyses of morphological and molecular data – and on a backbone taxonomy. To overcome problems associated with both the size of the group and the comparative paucity of available information, our analysis was made at the genus level (requiring synonymizing taxa at different levels across the trees) and used Safe Taxonomic Reduction to remove especially poorly known species. The final supertree contained 401 genera, making it the most comprehensive phylogenetic estimate yet published for the group. Interrelationships among the families are well resolved. Gyrinidae constitute the basal sister group, Haliplidae appear as the sister taxon of Geadephaga+ Dytiscoidea, Noteridae are the sister group of the remaining Dytiscoidea, Amphizoidae and Aspidytidae are sister groups, and Hygrobiidae forms a clade with Dytiscidae. Resolution within the species-rich Dytiscidae is generally high, but some relations remain unclear. Trachypachidae are the sister group of Carabidae (including Rhysodidae), in contrast to a proposed sister-group relationship between Trachypachidae and Dytiscoidea. Carabidae are only monophyletic with the inclusion of a non-monophyletic Rhysodidae, but resolution within this megadiverse group is generally low. Non-monophyly of Rhysodidae is extremely unlikely from a morphological point of view, and this group remains the greatest enigma in adephagan systematics. -
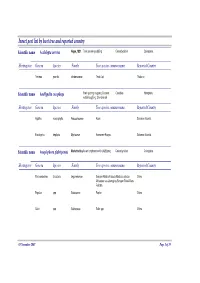
Insect Pest List by Host Tree and Reported Country
Insect pest list by host tree and reported country Scientific name Acalolepta cervina Hope, 1831 Teak canker grub|Eng Cerambycidae Coleoptera Hosting tree Genera Species Family Tree species common name Reported Country Tectona grandis Verbenaceae Teak-Jati Thailand Scientific name Amblypelta cocophaga Fruit spotting bug|eng Coconut Coreidae Hemiptera nutfall bug|Eng, Chinche del Hosting tree Genera Species Family Tree species common name Reported Country Agathis macrophylla Araucariaceae Kauri Solomon Islands Eucalyptus deglupta Myrtaceae Kamarere-Bagras Solomon Islands Scientific name Anoplophora glabripennis Motschulsky Asian longhorn beetle (ALB)|eng Cerambycidae Coleoptera Hosting tree Genera Species Family Tree species common name Reported Country Paraserianthes falcataria Leguminosae Sengon-Albizia-Falcata-Molucca albizia- China Moluccac sau-Jeungjing-Sengon-Batai-Mara- Falcata Populus spp. Salicaceae Poplar China Salix spp. Salicaceae Salix spp. China 05 November 2007 Page 1 of 35 Scientific name Aonidiella orientalis Newstead, Oriental scale|eng Diaspididae Homoptera 1894 Hosting tree Genera Species Family Tree species common name Reported Country Lovoa swynnertonii Meliaceae East African walnut Cameroon Azadirachta indica Meliaceae Melia indica-Neem Nigeria Scientific name Apethymus abdominalis Lepeletier, Tenthredinidae Hymenoptera 1823 Hosting tree Genera Species Family Tree species common name Reported Country Other Coniferous Other Coniferous Romania Scientific name Apriona germari Hope 1831 Long-horned beetle|eng Cerambycidae