SMDP Medtech 22-26 Sept
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mouth Rinses
Mouth Rinses We may recommend a mouth rinse to you for one or more of the following reasons: Periodontal Disease, Gingivitis, Halitosis (bad breath), sensitive teeth, decay or increased risk of decay, dry mouth, minor mouth sore or irritation. Red= RX only Blue= Available through your dentist only Green= Over the counter *Common side effects found with many mouth rinses include the possibility of staining. We still encourage you to use the rinse that treats your problem because none of the stains these rinses cause are permanent. With good oral hygiene on your part, using your bleaching trays and bleach as needed, you can keep the staining to a minimum. If there is any staining that you can’t prevent, it can always be easily removed by your hygienist at your next cleaning. If you have periodontal disease or Gingivitis, we may recommend an antimicrobial/antiseptic type rinse. These rinses kill germs, reduce plaque, redness, swelling and bleeding when used in conjunction with proper brushing and flossing. Some common examples of antiseptic rinses are: Peridex or Perioguard oral rinses are available by prescription only. Active ingredient is chlorhexidine gluconate .12%. These also contain alcohol 11.6%. Can cause staining of the teeth*, taste alteration and dry mouth. Periomed Oral rinse is available through your dentist only. Active ingredient is stannous fluoride .63%. It is alcohol free. Periomed provides antimicrobial activity for up to 8 hours. Also promotes enamel remineralization, and helps reduce sensitivity. Can cause staining* and dry mouth. Listerine Antiseptic: Active ingredient is alcohol 26.9% in the original gold Listerine, and 21.6% in the other flavors. -

Women in Business Awards Luncheon at the Hotel Irvine, Where Aston Martin Americas President Laura Schwab Delivered the Keynote Address
10.5.20 SR_WIB.qxp_Layout 1 10/2/20 12:14 PM Page 29 WOMEN IN BUSINESS NOMINEES START ON PAGE B-60 INSIDE 2019 WINNERS GO BIG IN IRVINE, LAND NEW PARTNERS, INVESTMENTS PAGE 30 PRESENTED BY DIAMOND SPONSOR PLATINUM SPONSORS GOLD SPONSOR SILVER SPONSORS 10.5.20 SR_WIB.qxp_Layout 1 10/2/20 1:36 PM Page 30 30 ORANGE COUNTY BUSINESS JOURNAL www.ocbj.com OCTOBER 5, 2020 Winning Execs Don’t Rest on Their Laurels $1B Cancer Center Underway; Military Wins; Spanish Drug Investment Orange County’s business community last year celebrated the Business Journal’s 25th annual Women in Business Awards luncheon at the Hotel Irvine, where Aston Martin Americas President Laura Schwab delivered the keynote address. The winners, selected from 200 nominees, have not been resting on their laurels, even in the era of the coronavirus. Here are updates on what the five winners have been doing. —Peter J. Brennan Avatar Partners City of Hope Shortly after Marlo Brooke won the Busi- (AR) quality assurance solution for the U.S. As president of the City of Hope Orange employees down from Duarte. Area univer- ness Journal’s award for co-founding Hunt- Navy for aircraft wiring maintenance for the County, Annette Walker is orchestrating a sities could partner with City of Hope. ington Beach-based Avatar Partners Inc., Naval Air Systems Command’s Boeing V- $1 billion project to build one of the biggest, While the larger campus near the Orange she was accepted into the Forbes Technol- 22 Osprey aircraft. and scientifically advanced, cancer research County Great Park is being built, Walker in ogy Council, an invitation-only community Then the Air Force is using Avatar’s solu- centers in the world. -

2015 Annual Report
ANNUAL REPORT 2015 MARCH 2016 TO OUR SHAREHOLDERS ALEX GORSKY Chairman, Board of Directors and Chief Executive Officer This year at Johnson & Johnson, we are proud this aligned with our values. Our Board of WRITTEN OVER to celebrate 130 years of helping people Directors engages in a formal review of 70 YEARS AGO, everywhere live longer, healthier and happier our strategic plans, and provides regular OUR CREDO lives. As I reflect on our heritage and consider guidance to ensure our strategy will continue UNITES & our future, I am optimistic and confident in the creating better outcomes for the patients INSPIRES THE long-term potential for our business. and customers we serve, while also creating EMPLOYEES long-term value for our shareholders. OF JOHNSON We manage our business using a strategic & JOHNSON. framework that begins with Our Credo. Written OUR STRATEGIES ARE BASED ON over 70 years ago, it unites and inspires the OUR BROAD AND DEEP KNOWLEDGE employees of Johnson & Johnson. It reminds OF THE HEALTH CARE LANDSCAPE us that our first responsibility is to the patients, IN WHICH WE OPERATE. customers and health care professionals who For 130 years, our company has been use our products, and it compels us to deliver driving breakthrough innovation in health on our responsibilities to our employees, care – from revolutionizing wound care in communities and shareholders. the 1880s to developing cures, vaccines and treatments for some of today’s most Our strategic framework positions us well pressing diseases in the world. We are acutely to continue our leadership in the markets in aware of the need to evaluate our business which we compete through a set of strategic against the changing health care environment principles: we are broadly based in human and to challenge ourselves based on the health care, our focus is on managing for the results we deliver. -

Johnson & Johnson
JOHNSON & JOHNSON FORM 10-K (Annual Report) Filed 02/22/13 for the Period Ending 12/30/12 Address ONE JOHNSON & JOHNSON PLZ NEW BRUNSWICK, NJ 08933 Telephone 732-524-2455 CIK 0000200406 Symbol JNJ SIC Code 2834 - Pharmaceutical Preparations Industry Biotechnology & Drugs Sector Healthcare Fiscal Year 12/12 http://www.edgar-online.com © Copyright 2013, EDGAR Online, Inc. All Rights Reserved. Distribution and use of this document restricted under EDGAR Online, Inc. Terms of Use. UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 FORM 10-K ANNUAL REPORT PURSUANT TO SECTION 13 OF THE SECURITIES EXCHANGE ACT OF 1934 For the fiscal year ended December 30, 2012 Commission file number 1-3215 JOHNSON & JOHNSON (Exact name of registrant as specified in its charter) New Jersey 22-1024240 (State of incorporation) (I.R.S. Employer Identification No.) One Johnson & Johnson Plaza New Brunswick, New Jersey 08933 (Address of principal executive offices) (Zip Code) Registrant’s telephone number, including area code: (732) 524-0400 SECURITIES REGISTERED PURSUANT TO SECTION 12(b) OF THE ACT Title of each class Name of each exchange on which registered Common Stock, Par Value $1.00 New York Stock Exchange Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes No Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Exchange Act. Yes No Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Exchange Act during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. -

1 Brief Report: the Virucidal Efficacy of Oral Rinse Components Against SARS-Cov-2 in Vitro Evelina Statkute1†, Anzelika Rubin
bioRxiv preprint doi: https://doi.org/10.1101/2020.11.13.381079; this version posted November 13, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-ND 4.0 International license. Brief Report: The Virucidal Efficacy of Oral Rinse Components Against SARS-CoV-2 In Vitro Evelina Statkute1†, Anzelika Rubina1†, Valerie B O’Donnell1, David W. Thomas2† Richard J. Stanton1† 1Systems Immunity University Research Institute, Division of Infection & Immunity, School of Medicine, Heath Park, Cardiff, CF14 4XN 2Advanced Therapies Group, School of Dentistry, Cardiff University, Heath Park, Cardiff CF14 4XY, UK †These authors contributed equally * Correspondence: [email protected], [email protected] Running title: Virucidal Activity of Mouthwashes Keywords: SARS-CoV2, mouthwash, lipid, envelope Disclosure: Venture Life Group plc provided information on mouthwash formulations employed in the study, but had no role in funding, planning, execution, analysis or writing of this study. A separate study funded to Cardiff University by Venture Life Group is assessing in vivo efficacy of CPC in patients with COVID19. The investigators declare no direct conflicts exist. 1 bioRxiv preprint doi: https://doi.org/10.1101/2020.11.13.381079; this version posted November 13, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-ND 4.0 International license. -

Manufacturers and Wholesalers Street
Nevada AB128 Code of Conduct Compliant Companies Manufacturers and Wholesalers Street City ST Zip 10 Edison Street LLC 13 Edison Street LLC Abbott Diabetes Care Division Abbott Diagnostic Division Abbott Electrophysiology (including Kalila Medical 2- 2016)) Abbott Laboratories 100 Abbott Park Road, Dept. EC10, Bldg. APGA-2 Abbott Park IL 60064 Abbott Medical Optics Abbott Molecular Division Abbott Nutrition Products Division Abbott Vascular Division (includes Tendyne 9-2015) AbbVie, Inc. 1 N. Waukegan Road North Chicago IL 60064 Acadia Phamaceuticals 3611 Valley Centre Drive, Suite 300 San Diego CA 92130 Accelero Health Partners, LLC Acclarent, Inc. 1525-B O'Brien Dr. Menlo Park CA 94025 Accuri Cyometers, Inc. Ace Surgical Supply, Inc. 1034 Pearl St. Brockton MA 02301 Acorda Therapeutics, Inc. 420 Sawmill River Road Ardsley NY 10532 AcriVet, Inc. Actavis W.C. Holding, Inc. Morris Corporate Center III, 400 Interpace Parkway Parsippany NJ 07054 Actavis , Inc. Actelion Pharmaceuticals US, Inc. 5000 Shoreline Court, Suite 200 S. San Francisco CA 94080 Activis 400 Interpace parkway Parsippany NJ 07054 A-Dec, Inc. 2601 Crestview Dr. Newberg OR 97132 Advanced Respiratory, Inc. Advanced Sterilization Products 33 Technology Drive Irvine CA 92618 Advanced Vision Research, Inc., dba Akorn Consumer Health Aegerion Pharmaceuticals, Inc. 101 Main Street, Suite 1850 Cambridge MA 02142 Aesculap Implant Systems, Inc. Aesculap, Inc. 3773 Corporate Parkway Center Valley PA 18034 Aesthera Corporation Afaxys, Inc. PO Box 20158 Charleston SC 29413 AGMS, Inc. Akorn (New Jersey) Inc. Page 1 of 23 Pages 2/15/2017 Nevada AB128 Code of Conduct Compliant Companies Akorn AG (formerly Excelvision AG) Akorn Animal Health, Inc. -
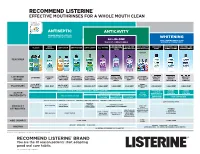
Listerine Floss Product Chart EN V9black
RECOMMEND LISTERINE® EFFECTIVE MOUTHRINSES FOR A WHOLE MOUTH CLEAN® 3 ANTISEPTIC ANTICAVITY 2X MORE HEALTHY SITES VS. JUST BRUSHING & FLOSSING WHITENING ALL-IN-ONE ONLY LEADING BRAND THAT THE MOST COMPLETE RINSE WHITENS & STRENGTHENS ALL-IN-ONE ZERO ALL-IN-ONE ANTI-CAVITY PEROXIDE WHITENS AND WHITENS AND CLASSIC ANTI-STAIN ANTI-TARTAR ANTI-CAVITY ALL-IN-ONE FOR SENSITIVE ALCOHOL TEETH ZERO ALCOHOL ZERO ALCOHOL FREE STRENGTHENS STRENGTHENS METERED DOSING FOR KIDS FEATURES ® LISTERINE LISTERINE LISTERINE LISTERINE LISTERINE LISTERINE LISTERINE LISTERINE LISTERINE LISTERINE LISTERINE LISTERINE LISTERINE TOTAL CARE HEALTHY HEALTHY HEALTHY ULTRACLEAN ULTRACLEAN ULTRACLEAN TOTAL CARE SMART RINSE ZERO TOTAL CARE FOR SENSITIVE WHITE™ WHITE™ WHITE™ BRAND ANTI-STAIN ANTI-TARTAR ANTI-CAVITY TEETH ZERO FOR KIDS GENTLE RESTORING VIBRANT COOL MINT BERRY FRESHBURST ARCTIC MINT BUBBLE GUM FLAVOURS MILD MINT COOL MINT FRESHBURST CLEAN MINT CLEAN MINT MILD MINT CLEAN MINT CLEAN MINT CLEAN MINT ORIGINAL COOL CITRUS MINT EUCALYPTOL 0.091% W/V MENTHOL 0.042% W/V THYMOL 0.063% W/V SODIUM FLUORIDE SODIUM SODIUM 0.02% W/W FLUORIDE FLUORIDE ACTIVE SODIUM FLUORIDE 0.022% W/V SODIUM TETRAPOTASSIUM 0.022% W/V 0.02% W/W FLUORIDE PYROPHOSPHATE INGREDIENTS ZINC CHLORIDE 0.09% W/V ZINC CHLORIDE POTASSIUM ZINC CHLORIDE 0.022% W/V PENTASODIUM HYDROGEN PEROXIDE 0.09% W/V NITRATE 2.4% W/V 0.09% W/V TRIPHOSPHATE KILLS UP TO 99.9% OF GERMS IN YOUR MOUTH PREVENTS & REDUCES GINGIVITIS PREVENTS & REDUCES PLAQUE SAFELY WHITENS TEETH† PREVENTS CAVITIES MILD FLAVOUR HELPS KEEP PREVENTS CAVITIES PRODUCT TEETH WHITE STRENGTHENS STRENGTHENS TOOTH ENAMEL* 2X BETTER TOOTH ENAMEL CAVITY ATTRIBUTES PROTECTION VS. -

Towards the Creation of Polymer Composites Which Can Be
TOWARDS THE CREATION OF POLYMER COMPOSITES WHICH CAN BE REFILLED WITH ANTIBIOTICS AFTER IMPLANTATION FOR INFECTION TREATMENT By ERIKA LEAH CYPHERT Submitted in partial fulfillment of the requirements For the degree of Doctor of Philosophy Dissertation Advisor: Horst von Recum, Ph.D. Department of Biomedical Engineering CASE WESTERN RESERVE UNIVERSITY January 2021 CASE WESTERN RESERVE UNIVERSITY SCHOOL OF GRADUATE STUDIES We hereby approve the thesis/dissertation of Erika Leah Cyphert Candidate for the Doctor of Philosophy degree*. (signed) Steven Eppell, Ph.D. (chair of committee) Horst von Recum, Ph.D. Eben Alsberg, Ph.D. Agata Exner, Ph.D. Jonathan Pokorski, Ph.D. (date) September 25, 2020 *We also certify that written approval has been obtained for any proprietary material contained therein. 2 To my grandparents with love – Phil and Ann Cyphert Ted and Dorothy Lippold Florence Miller 3 TABLE OF CONTENTS TABLE OF CONTENTS………………………………………………………………..4 LIST OF TABLES…………………………………………………………………..….10 LIST OF FIGURES…………………………………………………………………….13 LIST OF ABBREVIATIONS……………………………………………………….…21 ACKNOWLEDGEMENTS……………………………………………………………24 ABSTRACT…………………………………………………………………………..…27 CHAPTER 1: DIAGNOSIS AND BIOMATERIAL-BASED TREATMENTS FOR PERIPROSTHETIC JOINT INFECTIONS………………………………………….29 1.1. LIMITATIONS OF CLINICAL TREATMENT OF PERIPROSTHETIC INFECTION…………………………………………………………………30 1.1.1. INTRODUCTION…………………………………………….…30 1.1.2. ISOLATION OF MICROBIAL ORGANISMS………………....33 1.1.3. POSSIBLE UNDERLYING PATIENT COMORBIDITIES……33 1.1.4. BIOFILM FORMATION AND BACTERIAL RESISTANCE…34 4 1.1.5. REVISION PROCEDURES/INITIAL TREATMENT FAILURES....................................................................................36 1.1.6. SUMMARY……………………………………………………...37 1.2. NOVEL TREATMENT MODALITIES FOR PJIS………………………...38 1.2.1. LIMITATIONS WITH TRADITIONAL ANTIBIOTIC-LADEN PMMA BONE CEMENT……………………………………..…38 1.2.2. COMMERCIALLY AVAILABLE ALTERNATIVE BIOMATERIALS FOR ANTIBIOTIC-LADEN PMMA BONE CEMENT………………………………………………………...40 1.2.3. -

July 21, 2021
1 2nd Quarter 2021 Earnings Call July 21, 2021 Cautionary Note on Forward-looking Statements This presentation contains “forward-looking statements” as defined in the Private Securities Litigation Reform Act of 1995 regarding, among other things: future operating and financial performance, product development, market position and business strategy. The reader is cautioned not to rely on these forward-looking statements. These statements are based on current expectations of future events. If underlying assumptions prove inaccurate or known or unknown risks or uncertainties materialize, actual results could vary materially from the expectations and projections of Johnson & Johnson. Risks and uncertainties include, but are not limited to: risks related to the impact of the COVID-19 global pandemic, such as the scope and duration of the outbreak, government actions and restrictive measures implemented in response, material delays and cancellations of medical procedures, supply chain disruptions and other impacts to the business, or on the Company’s ability to execute business continuity plans, as a result of the COVID-19 pandemic; economic factors, such as interest rate and currency exchange rate fluctuations; competition, including technological advances, new products and patents attained by competitors; challenges inherent in new product research and development, including uncertainty of clinical success and obtaining regulatory approvals; uncertainty of commercial success for new and existing products; challenges to patents; the impact -

Johnson & Johnson Reports 2010 Second-Quarter Results
Johnson & Johnson Reports 2010 Second-Quarter Results: Sales of $15.3 Billion Increased 0.6% Versus 2009 Second-Quarter; EPS was $1.23 Excluding Special Items, 2010 Second-Quarter EPS was $1.21, an increase of 5.2%* NEW BRUNSWICK, N.J., July 20, 2010 /PRNewswire via COMTEX News Network/ -- Johnson & Johnson (NYSE: JNJ) today announced sales of $15.3 billion for the second quarter of 2010, an increase of 0.6% as compared to the second quarter of 2009. Operational results increased 0.1% and the positive impact of currency was 0.5%. Domestic sales declined 2.8%, while international sales increased 4.1%, reflecting operational growth of 3.0% and a positive currency impact of 1.1%. Net earnings and diluted earnings per share for the second quarter of 2010 were $3.4 billion and $1.23, respectively. Second- quarter 2010 net earnings included an after-tax gain of $67 million representing the net impact of litigation matters. Excluding this special item, net earnings for the current quarter were $3.4 billion and diluted earnings per share were $1.21, representing increases of 5.4% and 5.2%, respectively, as compared to the same period in 2009.* The Company updated its earnings guidance for full-year 2010 to $4.65 - $4.75 per share, which excludes the impact of special items. The Company's guidance now reflects the impact of the voluntary recalls announced earlier this year of certain over-the-counter medicines and the suspension of manufacturing at the McNeil Consumer Healthcare facility in Fort Washington, Pa., as well as unfavorable changes in foreign currency exchange rates. -
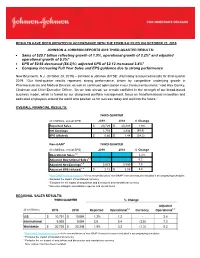
Sales of $20.7 Billion Reflecting Growth of 1.9%, Operational Growth of 3.2
RESULTS HAVE BEEN UPDATED IN ACCORDANCE WITH THE FORM 8-K FILED ON OCTOBER 23, 2019 JOHNSON & JOHNSON REPORTS 2019 THIRD-QUARTER RESULTS: • Sales of $20.7 billion reflecting growth of 1.9%, operational growth of 3.2%* and adjusted operational growth of 5.2%* • EPS of $0.66 decreased (54.2)%; adjusted EPS of $2.12 increased 3.4%* • Company increasing Full Year Sales and EPS guidance due to strong performance New Brunswick, N.J. (October 23, 2019) – Johnson & Johnson (NYSE: JNJ) today announced results for third-quarter 2019. “Our third-quarter results represent strong performance, driven by competitive underlying growth in Pharmaceuticals and Medical Devices, as well as continued optimization in our Consumer business,” said Alex Gorsky, Chairman and Chief Executive Officer. “As we look ahead, we remain confident in the strength of our broad-based business model, which is fueled by our disciplined portfolio management, focus on transformational innovation and dedicated employees around the world who position us for success today and well into the future.” OVERALL FINANCIAL RESULTS: THIRD QUARTER ($ in Millions, except EPS) 2019 2018 % Change Reported Sales $ 20,729 $ 20,348 1.9% Net Earnings 1,753 3,934 (55.4) EPS (diluted) $ 0.66 $ 1.44 (54.2) Non-GAAP* THIRD QUARTER ($ in Millions, except EPS) 2019 2018 % Change Operational Sales1,2 3.2% Adjusted Operational Sales1,3 5.2 Adjusted Net Earnings1,4 5,672 5,590 1.5 Adjusted EPS (diluted)1,4 $ 2.12 $ 2.05 3.4 1 Non-GAAP financial measure; refer to reconciliations of non-GAAP financial measures -

Department of Public Health 105 Cmr 720.000
105 CMR: DEPARTMENT OF PUBLIC HEALTH 105 CMR 720.000: LIST OF INTERCHANGEABLE DRUG PRODUCTS Section 720.001: Purpose 720.002: Citation 720.010: Scope and Application 720.020: Definitions Standards 720:040: Commission Review of Relevant Drug Products 720.050: List of Interchangeable Drug Products 720.060: Drug Products Excluded 720.070: Amendments to the Massachusetts List of Interchangeable Drugs Procedures for Amending List of Interchangeable Drug Products 720.080: Procedures for Amending the Massachusetts List of Interchangeable Drugs 720.081: Petition to Amend List of Interchangeable Drug Products 720.082: Commission Review of Petition 720.083: Notice of Public Comment Period 720.084: Commission Recommendation of Amendments to Department 720.090: Department Adoption of Amendments 720.100: Severability 720.200: Appendix A: List of Interchangeable Drugs 720.001: Purpose The purpose of 105 CMR 720.000 is to establish a drug formulary, or list of interchangeable drug products, for use by physicians, other practitioners, and pharmacists licensed to practice within the commonwealth, so that consumers of prescription drug products may realize cost savings by buying less expensive, safe drug products. 720.002: Citation 105 CMR 720.000 shall be known as the 105 CMR 720.000: Massachusetts List of Interchangeable Drug Products. 720.010: Scope and Application 105 CMR 720.000 establishes the list of interchangeable drug products from which a pharmacist must interchange a reasonably available less expensive drug product than that written, when a prescription written by a practitioner indicates "interchange". 105 CMR 720.000 also establishes criteria and procedures for inclusion of drug products on this list.