Laboratory Data Review for the Non-Chemist
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Use of Laboratory Equipement
USE OF LABORATORY EQUIPEMENT C. Laboratory Thermometers Most thermometers are based upon the principle that liquids expand when heated. Most common thermometers use mercury or colored alcohol as the liquid. These thermometers are constructed as that a uniform-diameter capillary tube surmounts a liquid reservoir. To calibrate a thermometer, one defines two reference points, normally the freezing point of water (0°C, 32°F) and the boiling point of water (100°C, 212°F) at 1 tam of pressure (1 tam = 760 mm Hg). Once these points are marked on the capillary, its length is then subdivided into uniform divisions called degrees. There are 100° between these two points on the Celsius (°C, or centigrade) scale and 180° between those two points on the Fahrenheit (°F) scale. °F = 1.8 °C + 32 The Thermometer and Its Calibration This section describes the proper technique for checking the accuracy of your thermometer. These measurements will show how measured temperatures (read from thermometer) compare with true temperatures (the boiling and freezing points of water). The freezing point of water is 0°C; the boiling point depends upon atmospheric pressure but at sea level it is 100°C. Option 1: Place approximately 50 mL of ice in a 250-mL beaker and cover the ice with distilled water. Allow about 15 min for the mixture to come to equilibrium and then measure and record the temperature of the mixture. Theoretically, this temperature is 0°C. Option 2: Set up a 250-mL beaker on a wire gauze and iron ring. Fill the beaker about half full with distilled water. -

Laboratory Glassware N Edition No
Laboratory Glassware n Edition No. 2 n Index Introduction 3 Ground joint glassware 13 Volumetric glassware 53 General laboratory glassware 65 Alphabetical index 76 Índice alfabético 77 Index Reference index 78 [email protected] Scharlau has been in the scientific glassware business for over 15 years Until now Scharlab S.L. had limited its sales to the Spanish market. However, now, coinciding with the inauguration of the new workshop next to our warehouse in Sentmenat, we are ready to export our scientific glassware to other countries. Standard and made to order Products for which there is regular demand are produced in larger Scharlau glassware quantities and then stocked for almost immediate supply. Other products are either manufactured directly from glass tubing or are constructed from a number of semi-finished products. Quality Even today, scientific glassblowing remains a highly skilled hand craft and the quality of glassware depends on the skill of each blower. Careful selection of the raw glass ensures that our final products are free from imperfections such as air lines, scratches and stones. You will be able to judge for yourself the workmanship of our glassware products. Safety All our glassware is annealed and made stress free to avoid breakage. Fax: +34 93 715 67 25 Scharlab The Lab Sourcing Group 3 www.scharlab.com Glassware Scharlau glassware is made from borosilicate glass that meets the specifications of the following standards: BS ISO 3585, DIN 12217 Type 3.3 Borosilicate glass ASTM E-438 Type 1 Class A Borosilicate glass US Pharmacopoeia Type 1 Borosilicate glass European Pharmacopoeia Type 1 Glass The typical chemical composition of our borosilicate glass is as follows: O Si 2 81% B2O3 13% Na2O 4% Al2O3 2% Glass is an inorganic substance that on cooling becomes rigid without crystallising and therefore it has no melting point as such. -

Sensitive Determination of PAH-DNA Adducts
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Matrix Design for Matrix-Assisted Laser Desorption Ionization: Sensitive Determination of PAH-DNA Adducts M. George, J. M. Y. Wellemans, R. L. Cerny, and M. L. Gross* Midwest Center for Mass Spectrometry, Department of Chemistry, University of Nebraska-Lincoln, Lincoln, Nebraska USA K. Li and E. L. Cavalieri Eppley institute for Research in Cancer and Allied Diseases, University of Nebraska Medical Center, 600 South 42nd Street, Omaha, Nebraska USA Two matrices, 4-phenyl-cw-cyanocinnamic acid (ICC) and 4-benzyloxy-cu-cyanocinnamic acid (BCC), were identified for the determination of polycyclic aromatic hydrocarbon @‘AH) adducts of DNA bases by matrix-assisted laser desorption ionization. These matrices were designed based on the concept that the matrix and the analyte should have structural similarities. PCC is a good matrix for the desorption of not only PA&modified DNA bases, but also PAHs themselves and their rnetabolites. Detections can be made at the femtomolar level. (1 Am Sot Mass Spectrum 1994, 5, 1021-1025) he success of matrix-assisted laser desorption quire sensitive detection methods at the low picomole ionization (MALDI) for the sensitive detection of and mid-femtomole level, respectively [6]. T an analyte depends on the nature of the matrix. One method for detection that also gives structural Rational design of a matrix requires understanding of information is fast-atom bombardment (FAB) coupled the mechanism of MALDI. The mechanism is not fully with tandem mass spectrometry [6-81. Unfortunately, understood. Nevertheless, empirical criteria for matrix the combination lacks sensitivity because there is in selection have been suggested 111. -

PML Brochure
PHYSICAL MEASUREMENT LABORATORY Gauging nature on all scales NIST.GOV/PML PHYSICAL MEASUREMENT LABORATORY (PML) The Physical Measurement Laboratory (PML), a major frequency, electricity, temperature, humidity, pressure operating unit of the National Institute of Standards and vacuum, liquid and gas flow, and electromag- and Technology (NIST), sets the definitive U.S. standards netic, optical, acoustic, and ionizing radiation. PML for nearly every kind of measurement in modern life, collaborates directly with industry, universities, profes- sometimes across more than 20 orders of magni- sional and standards-setting organizations, and other tude. PML is a world leader in the science of physical agencies of government to ensure accuracy and to measurement, devising procedures and tools that make solve problems. It also supports research in many continual progress possible. Exact measurements are fields of urgent national importance, such as manu- absolutely essential to industry, medicine, the research facturing, energy, health, law enforcement and community, and government. All of them depend on homeland security, communications, military defense, PML to develop, maintain, and disseminate the official electronics, the environment, lighting and display, standards for a wide range of quantities, including radiation, remote sensing, space exploration, length, mass, force and shock, acceleration, time and and transportation. IMPACTS ❱ Provides 700 kinds of calibration services ❱ Numerous special testing services NIST.GOV/PML | 2 NIST.GOV/PML -

Chemostat Culture for Yeast Experimental Evolution
Downloaded from http://cshprotocols.cshlp.org/ at Cold Spring Harbor Laboratory Library on August 9, 2017 - Published by Cold Spring Harbor Laboratory Press Protocol Chemostat Culture for Yeast Experimental Evolution Celia Payen and Maitreya J. Dunham1 Department of Genome Sciences, University of Washington, Seattle, Washington 98195 Experimental evolution is one approach used to address a broad range of questions related to evolution and adaptation to strong selection pressures. Experimental evolution of diverse microbial and viral systems has routinely been used to study new traits and behaviors and also to dissect mechanisms of rapid evolution. This protocol describes the practical aspects of experimental evolution with yeast grown in chemostats, including the setup of the experiment and sampling methods as well as best laboratory and record-keeping practices. MATERIALS It is essential that you consult the appropriate Material Safety Data Sheets and your institution’s Environmental Health and Safety Office for proper handling of equipment and hazardous material used in this protocol. Reagents Defined minimal medium appropriate for the experiment For examples, see Protocol: Assembly of a Mini-Chemostat Array (Miller et al. 2015). Ethanol (95%) Glycerol (20% and 50%; sterile) Yeast strain of interest Equipment Agar plates (appropriate for chosen strain) Chemostat array Assemble the apparatus as described in Miller et al. (2013) and Protocol: Assembly of a Mini-Chemostat Array (Miller et al. 2015). Cryo deep-freeze labels Cryogenic vials Culture tubes Cytometer (BD Accuri C6) Glass beads, 4 mm (sterile; for plating yeast cells) Glass cylinder Kimwipes 1Correspondence: [email protected] © 2017 Cold Spring Harbor Laboratory Press Cite this protocol as Cold Spring Harb Protoc; doi:10.1101/pdb.prot089011 559 Downloaded from http://cshprotocols.cshlp.org/ at Cold Spring Harbor Laboratory Library on August 9, 2017 - Published by Cold Spring Harbor Laboratory Press C. -

BROOKFIELD DIAL READING VISCOMETER with Electronic Drive
BROOKFIELD DIAL READING VISCOMETER with Electronic Drive Operating Instructions Manual No. M00-151-I0614 SPECIALISTS IN THE MEASUREMENT AND CONTROL OF VISCOSITY with offices in : Boston • Chicago • London • Stuttgart • Guangzhou BROOKFIELD ENGINEERING LABORATORIES, INC. 11 Commerce Boulevard, Middleboro, MA 02346 USA TEL 508-946-6200 or 800-628-8139 (USA excluding MA) FAX 508-946-6262 INTERNET http://www.brookfieldengineering.com TABLE OF CONTENTS I. INTRODUCTION .....................................................................................5 I.1 Components .......................................................................................................5 I.2 Utilities ................................................................................................................6 I.3 Specifications .....................................................................................................6 I.4 Set-Up ................................................................................................................7 I.5 IQ, OQ, PQ .........................................................................................................7 I.6 Safety Symbols and Precautions .......................................................................8 I.7 Cleaning .............................................................................................................8 II. GETTING STARTED ..............................................................................9 II.1 Operation ...........................................................................................................9 -

ELISA Plate Reader
applications guide to microplate systems applications guide to microplate systems GETTING THE MOST FROM YOUR MOLECULAR DEVICES MICROPLATE SYSTEMS SALES OFFICES United States Molecular Devices Corp. Tel. 800-635-5577 Fax 408-747-3601 United Kingdom Molecular Devices Ltd. Tel. +44-118-944-8000 Fax +44-118-944-8001 Germany Molecular Devices GMBH Tel. +49-89-9620-2340 Fax +49-89-9620-2345 Japan Nihon Molecular Devices Tel. +06-6399-8211 Fax +06-6399-8212 www.moleculardevices.com ©2002 Molecular Devices Corporation. Printed in U.S.A. #0120-1293A SpectraMax, SoftMax Pro, Vmax and Emax are registered trademarks and VersaMax, Lmax, CatchPoint and Stoplight Red are trademarks of Molecular Devices Corporation. All other trademarks are proprty of their respective companies. complete solutions for signal transduction assays AN EXAMPLE USING THE CATCHPOINT CYCLIC-AMP FLUORESCENT ASSAY KIT AND THE GEMINI XS MICROPLATE READER The Molecular Devices family of products typical applications for Molecular Devices microplate readers offers complete solutions for your signal transduction assays. Our integrated systems γ α β s include readers, washers, software and reagents. GDP αs AC absorbance fluorescence luminescence GTP PRINCIPLE OF CATCHPOINT CYCLIC-AMP ASSAY readers readers readers > Cell lysate is incubated with anti-cAMP assay type SpectraMax® SpectraMax® SpectraMax® VersaMax™ VMax® EMax® Gemini XS LMax™ ATP Plus384 190 340PC384 antibody and cAMP-HRP conjugate ELISA/IMMUNOASSAYS > nucleus Single addition step PROTEIN QUANTITATION cAMP > λEX 530 nm/λEM 590 nm, λCO 570 nm UV (280) Bradford, BCA, Lowry For more information on CatchPoint™ assay NanoOrange™, CBQCA kits, including the complete procedure for this NUCLEIC ACID QUANTITATION assay (MaxLine Application Note #46), visit UV (260) our web site at www.moleculardevices.com. -
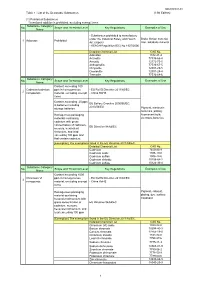
Table 1 : List of the Declarable Substances (1) Prohibited
DQ2000001-03 Table 1 : List of the Declarable Substances (13th Edition) (1) Prohibited Substances *Intentional addition is prohibited, excluding exempt items Substance Category/ No. Scope and Threshold Level Key Regulations Examples of Use Name - Substances prohibited to manufacture under the Industrial Safety and Health Brake friction material, 1 Asbestos Prohibited Act (Japan) filler, adiabatic material - REACH Regulation (EC) No 1907/2006 Detailed Chemical List CAS No. Asbestos 1332-21-4 Actinolite 77536-66-4 Amosite 12172-73-5 Anthophyllite 77536-67-5 Chrysotile 12001-29-5 Crocidolite 12001-28-4 Tremolite 77536-68-6 Substance Category/ No. Scope and Threshold Level Key Regulations Examples of Use Name Content exceeding 100 Cadmium/cadmium ppm in homogeneous - EU RoHS Directive 2011/65/EC 2 compounds material, excluding exempt - China RoHS items Content exceeding 20 ppm EU Battery Directive 2006/66/EC, in batteries including 2013/56/EU storage batteries Pigment, electronic materials, plating, Homogenous packaging fluorescent bulb, materials containing electrode,batteries cadmium with gross concentration of cadmium, EU Directive 94/62/EC mercury, hexavalent chromium, and lead exceeding 100 ppm and that contain cadmium [Exemption] The exemptions listed in the EU Directive 2011/65/EC. Detailed Chemical List CAS No. Cadmium 7440-43-9 Cadmium oxide 1306-19-0 Cadmium sulfide 1306-23-6 Cadmium chloride 10108-64-2 Cadmium sulfate 10124-36-4 Substance Category/ No. Scope and Threshold Level Key Regulations Examples of Use Name Content exceeding 1000 Chromium VI ppm in homogeneous - EU RoHS Directive 2011/65/EC 3 compounds material, excluding exempt - China RoHS items Homogenous packaging Pigment, catalyst, material containing plating, dye, surface hexavalent chromium with treatment gross concentration of EU Directive 94/62/EC cadmium, mercury, hexavalent chromium, and lead exceeding 100 ppm [Exemption] The exemptions listed in the EU Directive 2011/65/EC. -

Isoprene Photochemistry Over the Amazon Rainforest
Isoprene photochemistry over the Amazon rainforest Yingjun Liua, Joel Britob, Matthew R. Dorrisc, Jean C. Rivera-Riosc,d, Roger Secoe, Kelvin H. Batesf, Paulo Artaxob, Sergio Duvoisin Jr.g, Frank N. Keutscha,c,d, Saewung Kime, Allen H. Goldsteinh, Alex B. Guenthere,i, Antonio O. Manzij, Rodrigo A. F. Souzak, Stephen R. Springstonl, Thomas B. Watsonl, Karena A. McKinneya,1, and Scot T. Martina,m,1 aJohn A. Paulson School of Engineering and Applied Sciences, Harvard University, Cambridge, MA 02138; bDepartment of Applied Physics, University of São Paulo, São Paulo 05508, Brazil; cDepartment of Chemistry, University of Wisconsin-Madison, Madison, WI 53706; dDepartment of Chemistry and Chemical Biology, Harvard University, Cambridge, MA 02138; eDepartment of Earth System Science, University of California, Irvine, CA 92697; fDivision of Chemistry and Chemical Engineering, California Institute of Technology, Pasadena, CA 91125; gDepartment of Chemistry, Universidade do Estado do Amazonas, Manaus, AM 69050, Brazil; hDepartment of Environmental Science, Policy, and Management, University of California, Berkeley, CA 94720; iPacific Northwest National Laboratory, Richland, WA 99354; jInstituto Nacional de Pesquisas da Amazonia, Manaus, AM 69067, Brazil; kDepartment of Meteorology, Universidade do Estado do Amazonas, Manaus, AM 69050, Brazil; lDepartment of Environmental and Climate Sciences, Brookhaven National Laboratory, Upton, NY 11973; and mDepartment of Earth and Planetary Sciences, Harvard University, Cambridge, MA 02138 Edited by Mark H. Thiemens, University of California, San Diego, La Jolla, CA, and approved April 13, 2016 (received for review December 23, 2015) Isoprene photooxidation is a major driver of atmospheric chemistry forests like Amazonia where there are few anthropogenic NO over forested regions. -

Single-Molecule Fluorescence Detection
Proc. Nadl. Acad. Sci. USA Vol. 86, pp. 4087-4091, June 1989 Biophysics Single-molecule fluorescence detection: Autocorrelation criterion and experimental realization with phycoerythrin (laser-induced fluorescence/femtomolar sensitivity/fluorescence assays/phycoerythrin oligomers) KONAN PECK*, LUBERT STRYERt, ALEXANDER N. GLAZERt, AND RICHARD A. MATHIES* *Department of Chemistry, University of California, Berkeley, CA 94720; tDepartment of Cell Biology, Stanford University School of Medicine, Stanford, CA 94305; and tDepartment of Microbiology and Immunology, University of California, Berkeley, CA 94720 Contributed by Lubert Stryer, February 10, 1989 ABSTRACT A theory for single-molecule fluorescence ofindividual molecules. In this paper, we present a theory for detection is developed and then used to analyze data from single-molecule fluorescence detection and apply it in de- subpicomolar solutions of B-phycoerythrin (PE). The distri- tecting phycoerythrin at concentrations as low as 1 fM. bution of detected counts is the convolution of a Poissonian continuous background with bursts arising from the passage of individual fluorophores through the focused laser beam. The THEORY autocorrelation function reveals single-molecule events and Consider a solution of fluorophores flowing through a fo- provides a criterion for optimizing experimental parameters. cused laser beam. The emission from the illuminated volume The transit time of fluorescent molecules through the 120-fl consists of bursts of fluorescence from molecules passing imaged volume was 800 ps. The optimal laser power (32 mW through the beam superimposed on a continuous background at 514.5 nm) gave an incident intensity of 1.8 x 1023 due to Rayleigh and Raman scattering from the solvent (Fig. photons-cm 2.S-, corresponding to a mean time of 1.1 ns lA). -

State of Illinois Environmental Protection Agency Application for Environmental Laboratory Accreditation
State of Illinois Environmental Protection Agency Application for Environmental Laboratory Accreditation Attachment 6 Program: RCRA Field of Testing: Solid and Chemical Materials, Organic Matrix: Non Solid and Potable Chemical Accredited Water Materials Analyte: Status* Method (SW846): 1,2-Dibromo-3-chloropropane (DBCP) 8011 1,2-Dibromoethane (EDB) 8011 1,4-Dioxane 8015B 1-Butanol (n-Butyl alcohol) 8015B 1-Propanol 8015B 2-Butanone (Methyl ethyl ketone, MEK) 8015B 2-Chloroacrylonitrile 8015B 2-Methyl-1-propanol (Isobutyl alcohol) 8015B 2-Methylpyridine (2-Picoline) 8015B 2-Pentanone 8015B 2-Propanol (Isopropyl alcohol) 8015B 4-Methyl-2-pentanone (Methyl isobutyl ketone, 8015B MIBK) Acetone 8015B Acetonitrile 8015B Acrolein (Propenal) 8015B Acrylonitrile 8015B Allyl alcohol 8015B Crotonaldehyde 8015B Diesel range organics (DRO) 8015B Diethyl ether 8015B Ethanol 8015B Ethyl acetate 8015B Ethylene glycol 8015B Ethylene oxide 8015B Gasoline range organics (GRO) 8015B Hexafluoro-2-methyl-2-propanol 8015B * PI: Pending Initial Accreditation A: Accredited SP: Suspended WD: Accreditation Withdrawn Attachment 6: Page 1 of 62 Program: RCRA Field of Testing: Solid and Chemical Materials, Organic Matrix: Non Solid and Potable Chemical Accredited Water Materials Analyte: Status* Method (SW846): Hexafluoro-2-propanol 8015B Isopropyl benzene ((1-methylethyl) benzene) 8015B Methanol 8015B N-Nitrosodi-n-butylamine (N-Nitrosodibutylamine) 8015B o-Toluidine 8015B Paraldehyde 8015B Propionitrile (Ethyl cyanide) 8015B Pyridine 8015B t-Butyl alcohol 8015B -

I Fluorescent Detection of Chiral Amino Acids
i Fluorescent Detection of Chiral Amino Acids: A Micelle Approach Gengyu Du Yilong, Sichuan, China B.S. in Chemistry University of Science and Technology of China, Hefei, China, 2015 A Dissertation presented to the Graduate Faculty of the University of Virginia in Candidacy for the Degree of Doctor of Philosophy Department of Chemistry University of Virginia April 2020 ii Abstract Fluorescent Detection of Chiral Amino Acids: A Micelle Approach 1,1’-Bi-2-naphthol (BINOL)-based fluorescent probes have been developed as powerful tools for the molecular recognition of many chiral molecules. High sensitivity and enantioselectivity for chiral amino acids have been achieved in organic media, but the recognition in water media has been limited by their poor solubility in aqueous solution. By using a diblock copolymer PEG-PLLA, 3,3’-diformyl-BINOL was encapsulated into micelles to form a micelle probe. It allowed the detection of chiral lysine (Lys) both chemoselectively and enantioselectively in carbonate buffer solution. A limit of detection (LOD) of 848 nM and 2nd order fit of ee detection was obtained for D-Lys. The chemoselectivity was attributed to the selective reaction of Lys’s terminal amino groups with the probe. Series of monoformyl-BINOL with varying substituents were synthesized and engineered into micelle probes. These probes achieved specific detection of chiral tryptophan (Trp) both enantioselectively and chemoselectively in carbonate buffer solution, with LOD of 2.6 µM and 1st order fit of ee detection. A non-C2 symmetric molecule, 3,3’-diformyl-tetrahydro-BINOL, was fabricated into a micelle probe. It achieved specific detection of chiral His both enantioselectively and chemoselectively in carbonate buffer solution, with LOD of 107 nM and 2nd order fit of ee detection.