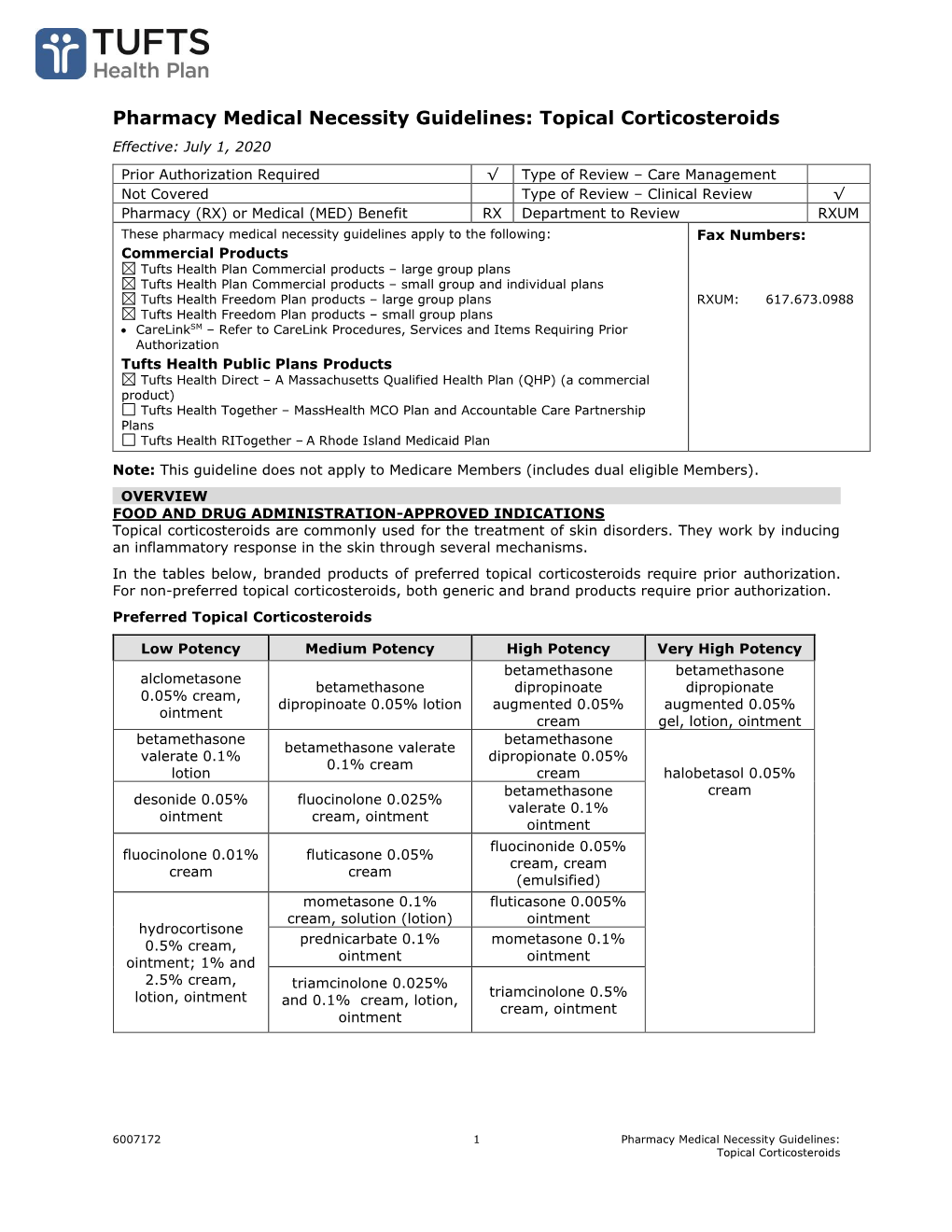
Pharmacy Medical Necessity Guidelines: Topical Corticosteroids
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

This Fact Sheet Provides Information to Patients with Eczema and Their Carers. About Topical Corticosteroids How to Apply Topic
This fact sheet provides information to patients with eczema and their carers. About topical corticosteroids You or your child’s doctor has prescribed a topical corticosteroid for the treatment of eczema. For treating eczema, corticosteroids are usually prepared in a cream or ointment and are applied topically (directly onto the skin). Topical corticosteroids work by reducing inflammation and helping to control an over-reactive response of the immune system at the site of eczema. They also tighten blood vessels, making less blood flow to the surface of the skin. Together, these effects help to manage the symptoms of eczema. There is a range of steroids that can be used to treat eczema, each with different strengths (potencies). On the next page, the potencies of some common steroids are shown, as well as the concentration that they are usually used in cream or ointment preparations. Using a moisturiser along with a steroid cream does not reduce the effect of the steroid. There are many misconceptions about the side effects of topical corticosteroids. However these treatments are very safe and patients are encouraged to follow the treatment regimen as advised by their doctor. How to apply topical corticosteroids How often should I apply? How much should I apply? Apply 1–2 times each day to the affected area Enough cream should be used so that the of skin according to your doctor’s instructions. entire affected area is covered. The cream can then be rubbed or massaged into the Once the steroid cream has been applied, inflamed skin. moisturisers can be used straight away if needed. -

DESONATE® (Desonide) Gel 0.05% for Topical Use Only • Systemic Absorption May Require Evaluation for HPA Axis Suppression Initial U.S
HIGHLIGHTS OF PRESCRIBING INFORMATION ----------------------- WARNINGS AND PRECAUTIONS------------------------ These highlights do not include all the information needed to use • Topical corticosteroids can produce reversible hypothalamic pituitary DESONATE Gel safely and effectively. See full prescribing information adrenal (HPA) axis suppression, Cushing's syndrome and unmask latent for DESONATE Gel. diabetes. (5.1) DESONATE® (desonide) Gel 0.05% for topical use only • Systemic absorption may require evaluation for HPA axis suppression Initial U.S. Approval: 1972 (5.1). • Modify use should HPA axis suppression develop (5.1) ---------------------------- INDICATIONS AND USAGE--------------------------- • Potent corticosteroids, use on large areas, prolonged use or occlusive use may increase systemic absorption (5.1) DESONATE is a corticosteroid indicated for the topical treatment of mild to moderate atopic dermatitis in patients 3 months of age and older. (1) • Local adverse reactions may include atrophy, striae, irritation, acneiform eruptions, hypopigmentation, and allergic contact dermatitis and may be ---------------------- DOSAGE AND ADMINISTRATION----------------------- more likely with occlusive use or more potent corticosteroids. (5.2, 5.4, 6) • Apply as a thin layer to the affected areas two times daily and rub in • Children may be more susceptible to systemic toxicity when treated with gently. (2) topical corticosteroids. (5.1, 8.4) • Therapy should be discontinued when control is achieved. (2) • If no improvement is seen within 4 weeks, reassessment of diagnosis ------------------------------ ADVERSE REACTIONS------------------------------- may be necessary. (2) The most common adverse reactions (incidence ≥ 1%) are headache and • Should not be used with occlusive dressings. (2) application site burning. (6) • Treatment beyond 4 consecutive weeks is not recommended. (2) To report SUSPECTED ADVERSE REACTIONS, contact LEO Pharma • For topical use only. -

Pricing Sheet
Trusted Service. Proven Savings. Pharmacy: (325) 704-5222 Fax: (325) 777-4819 17 Windmill Circle Suite B Abilene, TX 79606 bigcountry.clarxpharmacy.com SureScript ID: 1171544616 Dermatology Compounds UPDATED 9/3/2020 Medication QTY Price Fluorouracil/ Calcipotriene Cream 4.5/ 0.005% 30 g $55 Fluorouracil Cream 4.5% or 1.5% 30 g $50 Wart Peel Cream (Salicylic Acid/ Fluorouracil 17/ 2%) 30 g $45 Ivermectin Lotion 1.5% 30 g $45 Double Rosacea Cream (Metronidazole/ Ivermectin 1/ 1%) 30 g $55 Triple Rosacea Cream (Azelaic Acid/ Metronidazole/ Ivermectin 15/ 1/ 1%) 30 g $45 Azelaic Acid Cream 17% 30 g $45 Calcipotriene/ Clobetasol Cream 0.005/ 0.045% 60 g $85 Clioquinol/ Hydrocortisone Cream 1/ 1% 30 g $55 Tretinoin Micro Cream 0.033/ 0.055/ 0.09% 45 g $55 Clindamycin/ Tretinoin Micro Cream 1/0.03% 30 g $75 Triamcinolone/ Hydroquinone/ Tretinoin Cream 0.025/ 4/ 0.05% 30 g $55 Hydrocortisone/ Hydroquinone/ Tretinoin Cream 2.5/ 8/ 0.05% 30 g $55 Hydroquinone/ Kojic Acid Cream 12/ 6% or 10/ 3% 30 g $65 Hydroquinone/ Tretinoin Cream 4/ 0.05% 30 g $65 Squaric Acid Solution 0.1 / 0.01 / 0.001% 15 ml $85 Urea Cream 35% 90 g $45 Dapsone 6% Cream 60 g $55 Costmetic Numbing Oint. (Lidocain 23%/Tetracain 7%) 60g $35 General topical compound pricing for other compounds QTY Price Variations to the compounds above will fall into the pricing below. One Active Ingredient 30g $79 Two Active Ingredients 30g $89 Three Active Ingredients 30g $99 *This applies to most compounds (90%) not all, if exceptionally expensive we will call the office to discuss* All cash, no rebates or insurance to mess with! Medicare patients get medications at these great prices too! Free Delivery in 3-5 business days for compounds Page 1 Trusted Service. -

4. Antibacterial/Steroid Combination Therapy in Infected Eczema
Acta Derm Venereol 2008; Suppl 216: 28–34 4. Antibacterial/steroid combination therapy in infected eczema Anthony C. CHU Infection with Staphylococcus aureus is common in all present, the use of anti-staphylococcal agents with top- forms of eczema. Production of superantigens by S. aureus ical corticosteroids has been shown to produce greater increases skin inflammation in eczema; antibacterial clinical improvement than topical corticosteroids alone treatment is thus pivotal. Poor patient compliance is a (6, 7). These findings are in keeping with the demon- major cause of treatment failure; combination prepara- stration that S. aureus can be isolated from more than tions that contain an antibacterial and a topical steroid 90% of atopic eczema skin lesions (8); in one study, it and that work quickly can improve compliance and thus was isolated from 100% of lesional skin and 79% of treatment outcome. Fusidic acid has advantages over normal skin in patients with atopic eczema (9). other available topical antibacterial agents – neomycin, We observed similar rates of infection in a prospective gentamicin, clioquinol, chlortetracycline, and the anti- audit at the Hammersmith Hospital, in which all new fungal agent miconazole. The clinical efficacy, antibac- patients referred with atopic eczema were evaluated. In terial activity and cosmetic acceptability of fusidic acid/ a 2-month period, 30 patients were referred (22 children corticosteroid combinations are similar to or better than and 8 adults). The reason given by the primary health those of comparator combinations. Fusidic acid/steroid physician for referral in 29 was failure to respond to combinations work quickly with observable improvement prescribed treatment, and one patient was referred be- within the first week. -

(CD-P-PH/PHO) Report Classification/Justifica
COMMITTEE OF EXPERTS ON THE CLASSIFICATION OF MEDICINES AS REGARDS THEIR SUPPLY (CD-P-PH/PHO) Report classification/justification of medicines belonging to the ATC group D07A (Corticosteroids, Plain) Table of Contents Page INTRODUCTION 4 DISCLAIMER 6 GLOSSARY OF TERMS USED IN THIS DOCUMENT 7 ACTIVE SUBSTANCES Methylprednisolone (ATC: D07AA01) 8 Hydrocortisone (ATC: D07AA02) 9 Prednisolone (ATC: D07AA03) 11 Clobetasone (ATC: D07AB01) 13 Hydrocortisone butyrate (ATC: D07AB02) 16 Flumetasone (ATC: D07AB03) 18 Fluocortin (ATC: D07AB04) 21 Fluperolone (ATC: D07AB05) 22 Fluorometholone (ATC: D07AB06) 23 Fluprednidene (ATC: D07AB07) 24 Desonide (ATC: D07AB08) 25 Triamcinolone (ATC: D07AB09) 27 Alclometasone (ATC: D07AB10) 29 Hydrocortisone buteprate (ATC: D07AB11) 31 Dexamethasone (ATC: D07AB19) 32 Clocortolone (ATC: D07AB21) 34 Combinations of Corticosteroids (ATC: D07AB30) 35 Betamethasone (ATC: D07AC01) 36 Fluclorolone (ATC: D07AC02) 39 Desoximetasone (ATC: D07AC03) 40 Fluocinolone Acetonide (ATC: D07AC04) 43 Fluocortolone (ATC: D07AC05) 46 2 Diflucortolone (ATC: D07AC06) 47 Fludroxycortide (ATC: D07AC07) 50 Fluocinonide (ATC: D07AC08) 51 Budesonide (ATC: D07AC09) 54 Diflorasone (ATC: D07AC10) 55 Amcinonide (ATC: D07AC11) 56 Halometasone (ATC: D07AC12) 57 Mometasone (ATC: D07AC13) 58 Methylprednisolone Aceponate (ATC: D07AC14) 62 Beclometasone (ATC: D07AC15) 65 Hydrocortisone Aceponate (ATC: D07AC16) 68 Fluticasone (ATC: D07AC17) 69 Prednicarbate (ATC: D07AC18) 73 Difluprednate (ATC: D07AC19) 76 Ulobetasol (ATC: D07AC21) 77 Clobetasol (ATC: D07AD01) 78 Halcinonide (ATC: D07AD02) 81 LIST OF AUTHORS 82 3 INTRODUCTION The availability of medicines with or without a medical prescription has implications on patient safety, accessibility of medicines to patients and responsible management of healthcare expenditure. The decision on prescription status and related supply conditions is a core competency of national health authorities. -

Clinical Policy: Topical Agents: Corticosteroids
Clinical Policy: Topical Agents: Corticosteroids Reference Number: OH.PHAR.PPA.92 Effective Date: 01/01/2020 Revision Log Last Review Date: Line of Business: Medicaid See Important Reminder at the end of this policy for important regulatory and legal information. Description TOPICAL AGENTS: CORTICOSTEROIDS – LOW POTENCY NO PA REQUIRED “PREFERRED” PA REQUIRED “NON- PREFERRED” DESONIDE cream, ointment (generic of Desowen®) ALCLOMETASONE cream, ointment (generic of FLUOCINOLONE ACETONIDE 0.01% cream, solution Aclovate®) (generic of Synalar®) CAPEX® shampoo (fluocinolone acetonide) FLUOCINOLONE body oil, scalp oil (generic of Derma- DESONATE®gel (desonide) Smoothe/ FS®) DESONIDE lotion (generic of Desowen®) HYDROCORTISONE cream, lotion, ointment HYDROCORTISONE ACETATE WITH ALOE gel HYDROCORTISONE WITH UREA cream (generic of Carmol HC®) PANDEL® cream (hydrocortisone probutate) PEDIADERM HC® kit TOPICAL AGENTS: CORTICOSTEROIDS – MEDIUM POTENCY NO PA REQUIRED “PREFERRED” PA REQUIRED “NON--PREFERRED” BETAMETHASONE DIPROPIONATE-CALCIPOTRIENE BETAMETHASONE DIPROPIONATE lotion (generic of Ointment Diprolene®) BETAMETHASONE VALERATE cream, lotion (generic of CLOCORTOLONE PIVALATE (generic of Cloderm®) Valisone®) CORDRAN® tape (flurandrenolide) FLUTICASONE PROPIONATE cream, ointment (generic of DESOXIMETASONE cream, gel, ointment (generic of Cutivate®) Topicort®) MOMETASONE FUROATE cream, ointment, solution FLUOCINOLONE ACETONIDE 0.025% cream, ointment (generic of Elocon®) (generic of Synalar®) PREDNICARBATE cream (generic of Dermatop®) FLUTICASONE -

Steroids Topical
Steroids, Topical Therapeutic Class Review (TCR) September 18, 2020 No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, digital scanning, or via any information storage or retrieval system without the express written consent of Magellan Rx Management. All requests for permission should be mailed to: Magellan Rx Management Attention: Legal Department 6950 Columbia Gateway Drive Columbia, Maryland 21046 The materials contained herein represent the opinions of the collective authors and editors and should not be construed to be the official representation of any professional organization or group, any state Pharmacy and Therapeutics committee, any state Medicaid Agency, or any other clinical committee. This material is not intended to be relied upon as medical advice for specific medical cases and nothing contained herein should be relied upon by any patient, medical professional or layperson seeking information about a specific course of treatment for a specific medical condition. All readers of this material are responsible for independently obtaining medical advice and guidance from their own physician and/or other medical professional in regard to the best course of treatment for their specific medical condition. This publication, inclusive of all forms contained herein, is intended to be educational in nature and is intended to be used for informational purposes only. Send comments and suggestions to [email protected]. September -

Formulary Updates Effective January 1, 2021
Formulary Updates Effective January 1, 2021 Dear Valued Client, Please see the following lists of formulary updates that will apply to the HometownRx Formulary effective January 1 st , 2021. As the competition among clinically similar products increases, our formulary strategy enables us to prefer safe, proven medication alternatives and lower costs without negatively impacting member choice or access. Please note: Not all drugs listed may be covered under your prescription drug benefit. Certain drugs may have specific restrictions or special copay requirements depending on your plan. The formulary alternatives listed are examples of selected alternatives that are on the formulary. Other alternatives may be available. Members on a medication that will no longer be covered may want to talk to their healthcare providers about other options. Medications that do not have alternatives will be available at 100% member coinsurance . Preferred to Non -Preferred Tier Drug Disease State /Drug Class Preferred Alternatives ALREX Eye inflammation loteprednol (generic for LOTEMAX) APRISO 1 Gastrointestinal agent mesalamine (generic for APRISO) BEPREVE Eye allergies azelastine (generic for OPTIVAR) CIPRODEX 1 Ear inflammation ciprofloxacin-dexamethasone (generic for CIPRODEX) COLCRYS 1 Gout colchicine (generic for COLCRYS) FIRST -LANSOPRAZOLE Gastrointestinal agent Over-the-counter lansoprazole without a prescription FIRST -MOUTHWASH BLM Mouth inflammation lidocaine 2% viscous solution (XYLOCAINE) LOTEMAX 1 Eye inflammation loteprednol etabonate (generic -

Cream LOTRISONE® Lotion (Clotrimazole and Betamethasone Dipropionate)
LOTRISONE® Cream LOTRISONE® Lotion (clotrimazole and betamethasone dipropionate) FOR TOPICAL USE ONLY. NOT FOR OPHTHALMIC, ORAL, OR INTRAVAGINAL USE. NOT RECOMMENDED FOR PATIENTS UNDER THE AGE OF 17 YEARS AND NOT RECOMMENDED FOR DIAPER DERMATITIS. DESCRIPTION LOTRISONE® Cream and Lotion contain combinations of clotrimazole, a synthetic antifungal agent, and betamethasone dipropionate, a synthetic corticosteroid, for dermatologic use. Chemically, clotrimazole is 1–(o-chloro-α,α-diphenylbenzyl) imidazole, with the empirical formula C22H17CIN2, a molecular weight of 344.84, and the following structural formula: Clotrimazole is an odorless, white crystalline powder, insoluble in water and soluble in ethanol. Betamethasone dipropionate has the chemical name 9-fluoro-11β,17,21- trihydroxy-16β-methylpregna-1,4-diene-3,20-dione 17,21-dipropionate, with the empirical formula C28H37FO7, a molecular weight of 504.59, and the following structural formula: 1 LRN# 000370-LOS-MTL-USPI-1 Betamethasone dipropionate is a white to creamy white, odorless crystalline powder, insoluble in water. Each gram of LOTRISONE Cream contains 10 mg clotrimazole and 0.643 mg betamethasone dipropionate (equivalent to 0.5 mg betamethasone), in a hydrophilic cream consisting of purified water, mineral oil, white petrolatum, cetyl alcohol plus stearyl alcohol, ceteareth-30, propylene glycol, sodium phosphate monobasic monohydrate, and phosphoric acid; benzyl alcohol as preservative. LOTRISONE Cream may contain sodium hydroxide. LOTRISONE Cream is smooth, uniform, and white to off-white in color. Each gram of LOTRISONE Lotion contains 10 mg clotrimazole and 0.643 mg betamethasone dipropionate (equivalent to 0.5 mg betamethasone), in a hydrophilic base of purified water, mineral oil, white petrolatum, cetyl alcohol plus stearyl alcohol, ceteareth-30, propylene glycol, sodium phosphate monobasic monohydrate, and phosphoric acid; benzyl alcohol as a preservative. -

Medication List
Medication List Walgreens Plus™ members receive discounts on thousands of generic and brand-name medications included on this Medication List, which is divided into two sections, “Value Priced” Medications and “Discounted” Medications*. The price for a medication identified as “Value-Priced” is listed below: Get savings up to 85% off Cash Prices • 30-day-supply drugs cost $5 (tier 1), $10 (tier 2) or $15 (tier 3) on Atorvastatin (generic Lipitor) and • 90-day-supply drugs cost $10 (tier 1), $20 (tier 2) or $30 (tier 3) Rosuvastatin (generic Crestor) †† The Discounted Medications section lists the discounts offered to Walgreens Plus members on other generic and brand-name medications not included in the Value-Priced Medication section. The price for a medication is based on its tier and whether it is a 30-day or 90-day supply†. There may be an additional cost for quanities greater than those listed. This discount prescription pricing applies only to Walgreen Plus members on prescriptions purchased in select Walgreens stores that are not billed to insurance and/or used in combination with other health or pharmacy benefit programs. For further details, see your pharmacist or Walgreens.com/Plus. VALUE GENERICS NAPROXEN 250MG TAB 2 60 180 Antifungal NAPROXEN 500MG TAB 2 60 180 Quantity NAPROXEN 375MG TAB 2 60 180 Drug Name Tier 30 90 NAPROXEN DR 500MG TAB 3 60 180 FLUCONAZOLE 150MG TAB 2 1 3 TERBINAFINE 250MG TAB 2 30 90 Asthma Quantity Antiviral Drug Name Tier 30 90 Quantity ALBUTEROL 0.083% INH SOLN 25X3ML 2 75 225 Drug Name Tier 30 90 AMINOPHYLLINE -

1532 Corticosteroids
1532 Corticosteroids olone; Ultraderm; Malaysia: Synalar†; Mex.: Cortifung-S; Cortilona; Gr.: Lidex; Ital.: Flu-21†; Topsyn; Mex.: Topsyn; Norw.: Metosyn; Pharmacopoeias. In Br. Cremisona; Farmacorti; Flumicin; Fluomex; Fusalar; Lonason; Synalar; Philipp.: Lidemol; Lidex; Singapore: Lidex†; Spain: Klariderm†; Novoter; BP 2008 (Fluocortolone Hexanoate). A white or creamy-white, Norw.: Synalar; NZ: Synalar; Philipp.: Aplosyn; Cynozet; Synalar; Syntop- Switz.: To p s y m ; To p s y m i n ; UK: Metosyn; USA: Lidex; Vanos. ic; Pol.: Flucinar; Port.: Oto-Synalar N; Synalar; Rus.: Flucinar (Флуцинар); odourless or almost odourless, crystalline powder. It exhibits pol- Multi-ingredient: Austria: Topsym polyvalent; Ger.: Jelliproct; Topsym ymorphism. Practically insoluble in water and in ether; very Sinaflan (Синафлан); S.Afr.: Cortoderm; Fluoderm; Synalar; Singapore: polyvalent; Hung.: Vipsogal†; Israel: Comagis; Mex.: Topsyn-Y; Philipp.: Flunolone-V; Spain: Co Fluocin Fuerte; Cortiespec; Fluocid Forte; Fluoder- Lidex NGN; Spain: Novoter Gentamicina; Switz.: Mycolog N; Topsym slightly soluble in alcohol and in methyl alcohol; slightly soluble mo Fuerte; Flusolgen; Gelidina; Intradermo Corticosteroi†; Synalar; Synalar polyvalent; UK: Vipsogal. in acetone and in dioxan; sparingly soluble in chloroform. Pro- Rectal Simple; Swed.: Synalar; Switz.: Synalar; Thai.: Cervicum; Flu- ciderm†; Flunolone-V; Fulone; Supralan; Synalar; UK: Synalar; USA: Capex; tect from light. Derma-Smoothe/FS; DermOtic; Fluonid; Flurosyn†; Retisert; Synalar; Syn- emol; Venez.: Bratofil; Fluquinol Simple†; Neo-Synalar; Neoflu†. Fluocortin Butyl (BAN, USAN, rINNM) ⊗ Fluocortolone Pivalate (BANM, rINNM) ⊗ Multi-ingredient: Arg.: Adop-Tar†; Tri-Luma; Austria: Myco-Synalar; Procto-Synalar; Synalar N; Belg.: Procto-Synalar; Synalar Bi-Otic; Braz.: Butil éster de la fluocortina; Butylis Fluocortinas; Fluocortine Fluocortolone, pivalate de; Fluocortolone Trimethylacetate; Dermobel†; Dermoxin; Elotin; Fluo-Vaso; Neocinolon; Otauril†; Otocort†; Butyle; SH-K-203. -

Medical Review~ Clinical Review
CENTER FOR DRUG EVALUATION AND RESEARCH APPLICATION NUMBER: 21-978 MEDICAL REVIEW~ CLINICAL REVIEW Application Type NDA Submission Number 21-978 Submission Code 000 Letter Date November 18, 2005 Stamp Date November 28, 2005 PDUFA Goal Date September 21,2006 Reviewer Name Denise Cook, M.D. Review Completion Date 8/28/06 Established Name Desonide (Proposed) Trade Name .. Therapeutic Class Topical corticosteroid Applicant Connectics Priority Designatiop S Formulation Foam, 0.05% Dosing Regimen bid Indication F or the treatment of mild to moderate atopic dermatitis . Intended Population Age 3 months and above Clinical Review Denise Cook, M.D. NDA 21-978/ N-OOO _ loam, 0.05%/desonide foam, 0.05% Table of Contents 1 EXECUTIVE SUMMARy................................................................................................................................5 1.1 RECOMMENDATION ON REGULATORY ACTION ...........................................................................................5 1 .2 RECOMMENDATION ON POSTMARKETING ACTIONS. .............. ...... .... .... ........... ........ ..... ... ..... ...... ......... ........5 1.2. I Risk Management Activity................ ......... ..... ............... ..... ................... ........ ........ .... ....... ... ......... ........5 1.2.2 Required Phase 4 Commitments............................................................................................................5 1.2.3 Other Phase 4 Requests..........................................................................................................................5