Effect of Ecological Factors on the Accumulation of Phenolic Compounds in Iris Species from Latvia, Lithuania and Ukraine
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

(Iris Sibirica) Im Westlichen Bodenseegebiet 27-51 ©Staatl
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Carolinea - Beiträge zur naturkundlichen Forschung in Südwestdeutschland Jahr/Year: 2011 Band/Volume: 69 Autor(en)/Author(s): Peintinger Markus Artikel/Article: Verbreitung, Populationsdynamik und Vergesellschaftung der Sibirischen Schwertlilie (Iris sibirica) im westlichen Bodenseegebiet 27-51 ©Staatl. Mus. f. Naturkde Karlsruhe & Naturwiss. Ver. Karlsruhe e.V.; download unter www.zobodat.at carolinea, 69 (2011): 27-51, 4 Abb.; Karlsruhe, 15.12.2011 27 Verbreitung, Populationsdynamik und Vergesellschaftung der Sibirischen Schwertlilie (Iris sibirica) im westlichen Bodenseegebiet MARKUS PEINTINGER Kurzfassung alle Streuwiesen mit Iris sibirica befinden sich in Natur- Die Sibirische Schwertlilie (Iris sibirica) ist eine ty- schutzgebieten und werden aus Naturschutzgründen pische Art der Streuwiesen (Molinion caeruleae) am einmal im Jahr gemäht. Bodenseeufer. Der beobachtete Rückgang einiger Po- pulationen wurde zum Anlass genommen, um Verände- Abstract rungen in der Populationsgröße und der Artenzusam- Distribution, population dynamics and phytoso- mensetzung von Iris sibirica-Wiesen zu untersuchen. ciology of Iris sibirica in the western part of Lake Es wurde hauptsächlich drei Fragen nachgegangen: Constance 1. Wie ist die aktuelle Verbreitung von Iris sibirica und Iris sibirica is a characteristic species for calcareous hat sich der Bestand in den letzten hundert Jahren ver- fen meadows (Molinion caeruleae) at the shore of Lake ändert? 2. Gibt es einen langfristigen Populationstrend Constance. Since a decline of local populations was 1992-2008? 3. Hat sich die Artenzusammensetzung observed, a more detailed study was initiated to exa- der Streuwiesen mit Iris sibirica-Wiesen zwischen den mine changes in population size and species compo- zwei untersuchten Zeitperioden 1988-1993 und 2003- sition of Iris sibirica meadows. -
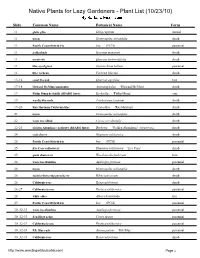
Native Plants for Lazy Gardeners - Plant List (10/23/10)
Native Plants for Lazy Gardeners - Plant List (10/23/10) Slide Common Name Botanical Name Form 11 globe gilia Gilia capitata annual 11 toyon Heteromeles arbutifolia shrub 11 Pacific Coast Hybrid iris Iris (PCH) perennial 11 goldenbush Isocoma menziesii shrub 11 scrub oak Quercus berberidifolia shrub 11 blue-eyed grass Sisyrinchium bellum perennial 11 lilac verbena Verbena lilacina shrub 13-16 coast live oak Quercus agrifolia tree 17-18 Howard McMinn man anita Arctostaphylos 'Howard McMinn' shrub 19 Philip Mun keckiella (RSABG Intro) Keckiella 'Philip Munz' ine 19 woolly bluecurls Trichostema lanatum shrub 19-20 Ray Hartman California lilac Ceanothus 'Ray Hartman' shrub 21 toyon Heteromeles arbutifolia shrub 22 western redbud Cercis occidentalis shrub 22-23 Golden Abundance barberry (RSABG Intro) Berberis 'Golden Abundance' (MAHONIA) shrub 2, coffeeberry Rhamnus californica shrub 25 Pacific Coast Hybrid iris Iris (PCH) perennial 25 Eve Case coffeeberry Rhamnus californica '. e Case' shrub 25 giant chain fern Woodwardia fimbriata fern 26 western columbine Aquilegia formosa perennial 26 toyon Heteromeles arbutifolia shrub 26 fuchsia-flowering gooseberry Ribes speciosum shrub 26 California rose Rosa californica shrub 26-27 California fescue Festuca californica perennial 28 white alder Alnus rhombifolia tree 29 Pacific Coast Hybrid iris Iris (PCH) perennial 30 032-33 western columbine Aquilegia formosa perennial 30 032-33 San Diego sedge Carex spissa perennial 30 032-33 California fescue Festuca californica perennial 30 032-33 Elk Blue rush Juncus patens '.l1 2lue' perennial 30 032-33 California rose Rosa californica shrub http://www weedingwildsuburbia com/ Page 1 30 032-3, toyon Heteromeles arbutifolia shrub 30 032-3, fuchsia-flowering gooseberry Ribes speciosum shrub 30 032-3, Claremont pink-flowering currant (RSA Intro) Ribes sanguineum ar. -

Seasonal Variation of Leaf Ecophysiological Traits of Iris Variegata Observed in Two Consecutive Years in Natural Habitats with Contrasting Light Conditions
Arch. Biol. Sci., Belgrade, 67(4), 1227-1236, 2015 DOI:10.2298/ABS150311099Z SEASONAL VARIATION OF LEAF ECOPHYSIOLOGICAL TRAITS OF IRIS VARIEGATA OBSERVED IN TWO CONSECUTIVE YEARS IN NATURAL HABITATS WITH CONTRASTING LIGHT CONDITIONS Uroš Živković, Danijela Miljković, Nataša Barišić Klisarić, Aleksej Tarasjev and Stevan Avramov* Institute for Biological Research “Siniša Stanković”, University of Belgrade, Belgrade, Serbia *Corresponding author: [email protected] Abstract: The amount and pattern of individual phenotypic responses to seasonal changes in environmental conditions were determined in clones of Iris variegata growing in differing light habitats. For the purpose of the study, 97 clonal plants of the rhizomatous herb I. variegata that experienced different light conditions in their two native habitats were selected: one along the top and slope of sand dunes and one in woodland understories. Two fully expanded leaves that had developed during spring, summer and fall in two consecutive years were sampled from each of these clones. Six leaf traits affecting the photosynthetic rate of a plant − morphological (specific leaf area), anatomical (stomatal density) and physiological (total chlorophyll concentration, chlorophyll a/chlorophyll b ratio, carotenoid concentration, chlorophyll a/carotenoid ratio) exhibited significant plastic responses in the two different light habitats. To test whether these traits differ between exposed and shaded habitats as well as during different vegetation periods, we used the repeated model analysis of variance (ANOVA). Results of the repeated ANOVA revealed statistically significant effects of year, habitat and period of vegetation season. Patterns of changes during growing seasons were year-specific for almost all analyzed traits. Key words: light conditions; seasonal change; I. -

Ecological Response of Some Iris L. Taxa (Iridaceae) in Turkey
Bangladesh J. Bot. 40(2): 177-184, 2011 (December) ECOLOGICAL RESPONSE OF SOME IRIS L. TAXA (IRIDACEAE) IN TURKEY 1 2 NEZAHAT KANDEMIR*, ALI ÇELIK AND ABDULKADIR SÜRÜCÜ Department of Biology, Education Faculty, Amasya University, Amasya-Turkey Key words: Iris taxa, Endemic, Rare, Ecological properties, Relationship Abstract Ecological relationships of some Iris taxa belonging to subgenera Hermodactyloides (I. danfordiae, I. histrio subsp. aintabensis, I. histrio subsp. histrio, I. reticulata, I. bakeriana, I. pamphylica) and Scorpiris (I. aucheri and I. persica) have been compared and relationships between taxa have been determined. These taxa are geophytes and flower in Spring. I. danfordiae, I. pamphylica and subsp. aintabensis are endemic to Turkey. I. reticulata and I. persica have widespread distribution while I. aucheri, subsp. aintabensis, subsp. histrio, I. pamphylica and I. bakeriana have restricted distribution in Turkey. Soil samples of the taxa were collected during flowering periods and physical and chemical properties (texture class, total salinity %, pH, CaCO3 %, organic matter %, N %, P kg/da, K kg/da, Ca , Mg, Mn, Cu, Fe and Zn ppm) were determined. The correlations between the soil analyses and taxa were evaluated using regression analysis. The P and CaCO3 values were found to be more effective than the other soil factors in the distributions of investigated taxa. Introduction Iris L. is one of the largest genus of Iridaceae family and comprises over 300 species in the world. They have been distributed in the Northern Hemisphere (Yu et al. 2009). Iris species have been used as ornamental plants in vegetative landscape of the parks and gardens in many countries since ancient times because of very beautiful and colorful flowers (specially in rock garden I. -

Weed Risk Assessment for Iris Pseudacorus L. (Iridaceae)
Weed Risk Assessment for Iris United States pseudacorus L. (Iridaceae) – Yellow Department of flag iris Agriculture Animal and Plant Health Inspection Service September 24, 2013 Version 1 Left: Iris pseudacorus flower. Right: A colony of Iris pseudacorus (source: Bugwood, 2013). Agency Contact: Plant Epidemiology and Risk Analysis Laboratory Center for Plant Health Science and Technology Plant Protection and Quarantine Animal and Plant Health Inspection Service United States Department of Agriculture 1730 Varsity Drive, Suite 300 Raleigh, NC 27606 Weed Risk Assessment for Iris pseudacorus Introduction Plant Protection and Quarantine (PPQ) regulates noxious weeds under the authority of the Plant Protection Act (7 U.S.C. § 7701-7786, 2000) and the Federal Seed Act (7 U.S.C. § 1581-1610, 1939). A noxious weed is defined as “any plant or plant product that can directly or indirectly injure or cause damage to crops (including nursery stock or plant products), livestock, poultry, or other interests of agriculture, irrigation, navigation, the natural resources of the United States, the public health, or the environment” (7 U.S.C. § 7701-7786, 2000). We use weed risk assessment (WRA)— specifically, the PPQ WRA model (Koop et al., 2012)—to evaluate the risk potential of plants, including those newly detected in the United States, those proposed for import, and those emerging as weeds elsewhere in the world. Because the PPQ WRA model is geographically and climatically neutral, it can be used to evaluate the baseline invasive/weed potential of any plant species for the entire United States or for any area within it. As part of this analysis, we use a stochastic simulation to evaluate how much the uncertainty associated with the analysis affects the model outcomes. -

Plants Species of Community Interest Identified in the Flora of the Transylvanian Plain (Mureş County)
Studia Universitatis “Vasile Goldiş”, Seria Ştiinţele Vieţii Vol. 27 issue 3, 2017, pp 209-214 © 2017 Vasile Goldis University Press (www.studiauniversitatis.ro) PLANTS SPECIES OF COMMUNITY INTEREST IDENTIFIED IN THE FLORA OF THE TRANSYLVANIAN PLAIN (MUREŞ COUNTY) Silvia OROIAN*1, Mihaela SĂMĂRGHIŢAN2, Corneliu TĂNASE3 1,3 University of Medicine and Pharmacy, Faculty of Pharmacy Tîrgu Mureş 2 Mureş County Museum, Department of Natural Sciences Tîrgu Mureş ABSTRACT: The purpose of this study is to present the species of community interest identified in the cormoflora of the Transylvanian Plain (Mureş County) as well as to verify in the field the existing chorological information, to select the endangered plant categories and to highlight the main populations of valuable species. Because of the location of the region, the diversity of the reliefs (hills, meadows), the various exhibitions and inclinations of the slopes, the Transylvanian Plain is distinguished by a great diversity of vegetal taxa (Oroian, Sămărghițan, 2014). The target species were the species of community interest listed in the Annexes to the Habitats Directive and those of O.U.G. no.57/2007. KEYWORDS: conservation status, Mureş county, Transylvania INTRODUCTION: taking in consideration authors experience and The Transylvanian Plain is represented by the previous personal field researches. central part of the Transylvanian Depression and extends on the territory of three counties: Bistriţa- RESULTS: Năsăud, Mureş and Cluj. In the territory of Mureş In the study area, seven species of community County, the Transylvanian Plain, situated to the north interest were identified. Of these: of the Mureş River, is a region formed by hills with an 4 species are LC (least concern) – Lowest average altitude of 400 m and is walled by the wide risk; does not qualify for a higher risk category. -

Morphological Peculiarities of Fruits of the Rare Species Iris Halophila Pall, I. Pumila L. and I. Hungarica Waldst. Et Kit
Plant Introduction, 85/86, 85–92 (2020) https://doi.org/10.46341/PI2020007 UDC 581.47:582.579.2(477-25) RESEARCH ARTICLE Morphological peculiarities of fruits of the rare species Iris halophila Pall, I. pumila L. and I. hungarica Waldst. et Kit. (Iridaceae Juss.) in the conditions of introduction in the meadow-steppe cultural phytocenosis V.V. Gritsenko M.M. Gryshko National Botanical Garden, National Academy of Sciences of Ukraine, Timiryazevska str. 1, 01014 Kyiv, Ukraine; [email protected] Received: 27.01.2020 | Accepted: 23.05.2020 | Published: 30.06.2020 Abstract The objective of this study was to analyze the morphological structure and to reveal common and distinguishing features of the fruit in rare steppe species Iris halophila, I. pumila and I. hungarica introduced in conditions of meadow-steppe cultural phytocenosis in the M.M. Gryshko National Botanical Garden, National Academy of Sciences of Ukraine (NBG). Material and methods. Fruits of I. halophila, I. pumila and I. hungarica were collected on the botanical- geographical plot “Steppes of Ukraine” of NBG during 2015–2019. Fruit parameters were measured using a regular ruler. Morphological terms are provided, according to Artyushenko & Fedorov (1986). Colors were determined by Bondartsev’s (1954) scale. Results. In all analyzed species, the fruit is a trimeric and trilocular loculicidal capsule with multi-seeded locules. This capsule is erect, straight, leathery, glabrous, opening by dehiscence from top to bottom along the dorsal veins of carpels. The morphological peculiarities of the fruits, which may be additional diagnostic characters of these species, are established. In particular, in I. halophila capsule is cylindrical, with the upper part elongated into the apical spout (long, thin, bent to the side). -

A HANDBOOK of GARDEN IRISES by W
A HANDBOOK OF GARDEN IRISES By W. R. DYKES, M.A., L.-ès-L. SECRETARY OF THE ROYAL HORTICULTURAL SOCIETY. AUTHOR OF "THE GENUS IRIS," ETC. CONTENTS. PAGE PREFACE 3 1 THE PARTS OF THE IRTS FLOWER AND PLANT 4 2 THE VARIOUS SECTIONS OF THE GENUS AND 5 THEIR DISTRIBUTION 3 THE GEOGRAPHICAL DISTRIBUTION OF THE VARIOUS 10 SECTIONS AND SPECIES AND THEIR RELATIVE AGES 4 THE NEPALENSIS SECTION 13 5 THE GYNANDRIRIS SECTION 15 6 THE RETICULATA SECTION 16 7 THE JUNO SECTION 23 8 THE XIPHIUM SECTION 33 9 THE EVANSIA SECTION 40 10 THE PARDANTHOPSIS SECTION 45 11 THE APOGON SECTION 46 — THE SIBIRICA SUBSECTION 47 — THE SPURIA SUBSECTION 53 — THE CALIFORNIAN SUBSECTION 59 — THE LONGIPETALA SUBSECTION 64 — THE HEXAGONA SUBSECTION 67 — MISCELLANEOUS BEARDLESS IRISES 69 12 THE ONCOCYCLUS SECTION 77 I. Polyhymnia, a Regeliocydus hybrid. 13 THE REGELIA SECTION 83 (I. Korolkowi x I. susianna). 14 THE PSEUDOREGELIA SECTION 88 15 THE POGONIRIS SECTION 90 16 GARDEN BEARDED IRISES 108 17 A NOTE ON CULTIVATION, ON RAISING 114 SEEDLINGS AND ON DISEASES 18 A TABLE OF TIMES OF PLANTING AND FLOWERING 116 19 A LIST OF SYNONYMS SOMETIMES USED IN 121 GARDENS This edition is copyright © The Goup for Beardless Irises 2009 - All Rights Reserved It may be distributed for educational purposes in this format as long as no fee (or other consideration) is involved. www.beardlessiris.org PREFACE TO THIS DIGITAL EDITION William Rickatson Dykes (1877-1925) had the advantage of growing irises for many years before writing about them. This Handbook published in 1924 represents the accumulation of a lifetime’s knowledge. -

Philip J. Savage, Jr
ISSUE 73 M4SNOU4 Philip J. Savage, Jr. May 8, 1917 — October 13, 2002 Phil Savnge, /r. , an icon m the Magnolia worhl, passed au ay last October of conr plica- lions from the West Nile virus. Phil, a renotvrred magnolin Irybcidrzer, specialized in breeding mngnolias that u&cre cold Irardy. Becnuse of this, nragnolia entirusiasts living in colder climalcs now have morry rrrorc choices thnn did Dennis Ledvh rn u&hen he bought Iris lrouse irr Green Bay, Wiscorrsin in thc Intr typos Plril hns left an errduring legaclt n&itlr tire mnny /Inc, cold-lurrdy hybrids Ire bred. Following arc set&eral rwni niscclrces fronr irrdividuals who toere deqviy in/luencerl by Phil mrd Iris u&ork u&ith mngrrolins. DENNIs LEDVINA WIUTES. Back in the late yos the landscaping around my new house consisted of three magnolias: two M. x sorrlarrgeana, and a M. x loebneri 'MerrilL' At the time, these were the only magnolias gener- ally available at local nurseries. As I watched these magnolias bloom each spring, I became more intrigued with their beautiful Bowers and began driv- ing around Green Bay to observe and admire some of the established trees. My admiration for the genus contin- ued to grow each year as I began col- lecting more information about these magnificent plants. One summer I was in the Detroit area and I decided to call this magnolia expert, Phil Savage, that I had read so much about. I can vividly remember calling Phil from a telephone booth on Telegraph Road and finding mysell, an unknown amateur, talking to a magnolia expert who from the first made me feel like a lifelong friend. -

AGS Seed List No 69 2020
Seed list No 69 2020-21 Garden Collected Seed 1001 Abelia floribunda 1057 Agrostemma githago 1002 Abies koreana 1058 Albuca canadensis (L. -

Qrno. 1 2 3 4 5 6 7 1 CP 2903 77 100 0 Cfcl3
QRNo. General description of Type of Tariff line code(s) affected, based on Detailed Product Description WTO Justification (e.g. National legal basis and entry into Administration, modification of previously the restriction restriction HS(2012) Article XX(g) of the GATT, etc.) force (i.e. Law, regulation or notified measures, and other comments (Symbol in and Grounds for Restriction, administrative decision) Annex 2 of e.g., Other International the Decision) Commitments (e.g. Montreal Protocol, CITES, etc) 12 3 4 5 6 7 1 Prohibition to CP 2903 77 100 0 CFCl3 (CFC-11) Trichlorofluoromethane Article XX(h) GATT Board of Eurasian Economic Import/export of these ozone destroying import/export ozone CP-X Commission substances from/to the customs territory of the destroying substances 2903 77 200 0 CF2Cl2 (CFC-12) Dichlorodifluoromethane Article 46 of the EAEU Treaty DECISION on August 16, 2012 N Eurasian Economic Union is permitted only in (excluding goods in dated 29 may 2014 and paragraphs 134 the following cases: transit) (all EAEU 2903 77 300 0 C2F3Cl3 (CFC-113) 1,1,2- 4 and 37 of the Protocol on non- On legal acts in the field of non- _to be used solely as a raw material for the countries) Trichlorotrifluoroethane tariff regulation measures against tariff regulation (as last amended at 2 production of other chemicals; third countries Annex No. 7 to the June 2016) EAEU of 29 May 2014 Annex 1 to the Decision N 134 dated 16 August 2012 Unit list of goods subject to prohibitions or restrictions on import or export by countries- members of the -

Population Genetic Structure of Iris Pumila L. in Ukraine: Effects of Habitat Fragmentation
ACTA BIOLOGICA CRACOVIENSIA Series Botanica 62/1: 51–61, 2020 10.24425/abcsb.2020.131665 POPULATION GENETIC STRUCTURE OF IRIS PUMILA L. IN UKRAINE: EFFECTS OF HABITAT FRAGMENTATION Olena Bublyk1 , Igor Andreev1* , Ivan Parnikoza1,2 , Viktor Kunakh1 1Institute of Molecular Biology and Genetics of NAS of Ukraine, Akad. Zabolotny str., 150, Kyiv, 03143, Ukraine 2National Antarctic Scientific Center of Ukraine, Boulevard Tarasa Shevchenka 16, 01601 Kyiv, Ukraine Received January 10, 2020; revision accepted April 17, 2020 Habitat fragmentation is one of serious threats to biodiversity of nature in today's world. The present study of a typical steppe species Iris pumila L. (Iridaceae) has analyzed the impacts of geographical isolation and population size on genetic diversity and population structure in conditions of habitat fragmentation. The key indices of population genetic variability calculated from the ISSR markers data were on average as follows: Shannon diversity index (S) – 0.188; unbiased Nei’s gene diversity (He) – 0.123; and the average measure of Jaccard’s genetic distances between individuals within populations – 58.4%. Although the largest population had significantly higher values of S and He, the small and marginal populations also showed a comparable level of variation. Most of the genetic variation of I. pumila was distributed within the populations. A strong correlation was found between Nei’s genetic distances and geographic distances between the populations. According to the Bayesian analysis, genetic structure of the populations was highly homogeneous; however, the presence of admixed genotypes indicated the possibility of gene flow between the populations at present. Keywords: conservation, genetic polymorphism, habitat fragmentation, ISSR markers, population genetics INTRODUCTION (Aguilar et al., 2008; Ewers and Didham, 2006).