Reprint with Additions Aims and Scope Topics in Geobiology Book Series
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Educators' Resource Guide
EDUCATORS' RESOURCE GUIDE Produced and published by 3D Entertainment Distribution Written by Dr. Elisabeth Mantello In collaboration with Jean-Michel Cousteau’s Ocean Futures Society TABLE OF CONTENTS TO EDUCATORS .................................................................................................p 3 III. PART 3. ACTIVITIES FOR STUDENTS INTRODUCTION .................................................................................................p 4 ACTIVITY 1. DO YOU Know ME? ................................................................. p 20 PLANKton, SOURCE OF LIFE .....................................................................p 4 ACTIVITY 2. discoVER THE ANIMALS OF "SECRET OCEAN" ......... p 21-24 ACTIVITY 3. A. SECRET OCEAN word FIND ......................................... p 25 PART 1. SCENES FROM "SECRET OCEAN" ACTIVITY 3. B. ADD color to THE octoPUS! .................................... p 25 1. CHristmas TREE WORMS .........................................................................p 5 ACTIVITY 4. A. WHERE IS MY MOUTH? ..................................................... p 26 2. GIANT BasKET Star ..................................................................................p 6 ACTIVITY 4. B. WHat DO I USE to eat? .................................................. p 26 3. SEA ANEMONE AND Clown FISH ......................................................p 6 ACTIVITY 5. A. WHO eats WHat? .............................................................. p 27 4. GIANT CLAM AND ZOOXANTHELLAE ................................................p -

CEPHALOPODS 688 Cephalopods
click for previous page CEPHALOPODS 688 Cephalopods Introduction and GeneralINTRODUCTION Remarks AND GENERAL REMARKS by M.C. Dunning, M.D. Norman, and A.L. Reid iving cephalopods include nautiluses, bobtail and bottle squids, pygmy cuttlefishes, cuttlefishes, Lsquids, and octopuses. While they may not be as diverse a group as other molluscs or as the bony fishes in terms of number of species (about 600 cephalopod species described worldwide), they are very abundant and some reach large sizes. Hence they are of considerable ecological and commercial fisheries importance globally and in the Western Central Pacific. Remarks on MajorREMARKS Groups of CommercialON MAJOR Importance GROUPS OF COMMERCIAL IMPORTANCE Nautiluses (Family Nautilidae) Nautiluses are the only living cephalopods with an external shell throughout their life cycle. This shell is divided into chambers by a large number of septae and provides buoyancy to the animal. The animal is housed in the newest chamber. A muscular hood on the dorsal side helps close the aperture when the animal is withdrawn into the shell. Nautiluses have primitive eyes filled with seawater and without lenses. They have arms that are whip-like tentacles arranged in a double crown surrounding the mouth. Although they have no suckers on these arms, mucus associated with them is adherent. Nautiluses are restricted to deeper continental shelf and slope waters of the Indo-West Pacific and are caught by artisanal fishers using baited traps set on the bottom. The flesh is used for food and the shell for the souvenir trade. Specimens are also caught for live export for use in home aquaria and for research purposes. -
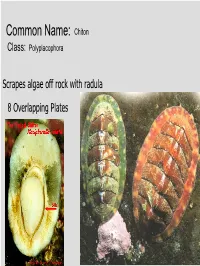
Common Name: Chiton Class: Polyplacophora
Common Name: Chiton Class: Polyplacophora Scrapes algae off rock with radula 8 Overlapping Plates Phylum? Mollusca Class? Gastropoda Common name? Brown sea hare Class? Scaphopoda Common name? Tooth shell or tusk shell Mud Tentacle Foot Class? Gastropoda Common name? Limpet Phylum? Mollusca Class? Bivalvia Class? Gastropoda Common name? Brown sea hare Phylum? Mollusca Class? Gastropoda Common name? Nudibranch Class? Cephalopoda Cuttlefish Octopus Squid Nautilus Phylum? Mollusca Class? Gastropoda Most Bivalves are Filter Feeders A B E D C • A: Mantle • B: Gill • C: Mantle • D: Foot • E: Posterior adductor muscle I.D. Green: Foot I.D. Red Gills Three Body Regions 1. Head – Foot 2. Visceral Mass 3. Mantle A B C D • A: Radula • B: Mantle • C: Mouth • D: Foot What are these? Snail Radulas Dorsal HingeA Growth line UmboB (Anterior) Ventral ByssalC threads Mussel – View of Outer Shell • A: Hinge • B: Umbo • C: Byssal threads Internal Anatomy of the Bay Mussel A B C D • A: Labial palps • B: Mantle • C: Foot • D: Byssal threads NacreousB layer Posterior adductorC PeriostracumA muscle SiphonD Mantle Byssal threads E Internal Anatomy of the Bay Mussel • A: Periostracum • B: Nacreous layer • C: Posterior adductor muscle • D: Siphon • E: Mantle Byssal gland Mantle Gill Foot Labial palp Mantle Byssal threads Gill Byssal gland Mantle Foot Incurrent siphon Byssal Labial palp threads C D B A E • A: Foot • B: Gills • C: Posterior adductor muscle • D: Excurrent siphon • E: Incurrent siphon Heart G F H E D A B C • A: Foot • B: Gills • C: Mantle • D: Excurrent siphon • E: Incurrent siphon • F: Posterior adductor muscle • G: Labial palps • H: Anterior adductor muscle Siphon or 1. -

Nautiloid Shell Morphology
MEMOIR 13 Nautiloid Shell Morphology By ROUSSEAU H. FLOWER STATEBUREAUOFMINESANDMINERALRESOURCES NEWMEXICOINSTITUTEOFMININGANDTECHNOLOGY CAMPUSSTATION SOCORRO, NEWMEXICO MEMOIR 13 Nautiloid Shell Morphology By ROUSSEAU H. FLOIVER 1964 STATEBUREAUOFMINESANDMINERALRESOURCES NEWMEXICOINSTITUTEOFMININGANDTECHNOLOGY CAMPUSSTATION SOCORRO, NEWMEXICO NEW MEXICO INSTITUTE OF MINING & TECHNOLOGY E. J. Workman, President STATE BUREAU OF MINES AND MINERAL RESOURCES Alvin J. Thompson, Director THE REGENTS MEMBERS EXOFFICIO THEHONORABLEJACKM.CAMPBELL ................................ Governor of New Mexico LEONARDDELAY() ................................................... Superintendent of Public Instruction APPOINTEDMEMBERS WILLIAM G. ABBOTT ................................ ................................ ............................... Hobbs EUGENE L. COULSON, M.D ................................................................. Socorro THOMASM.CRAMER ................................ ................................ ................... Carlsbad EVA M. LARRAZOLO (Mrs. Paul F.) ................................................. Albuquerque RICHARDM.ZIMMERLY ................................ ................................ ....... Socorro Published February 1 o, 1964 For Sale by the New Mexico Bureau of Mines & Mineral Resources Campus Station, Socorro, N. Mex.—Price $2.50 Contents Page ABSTRACT ....................................................................................................................................................... 1 INTRODUCTION -

First Records and Descriptions of Early-Life Stages of Cephalopods from Rapa Nui (Easter Island) and the Nearby Apolo Seamount
First records and descriptions of early-life stages of cephalopods from Rapa Nui (Easter Island) and the nearby Apolo Seamount By Sergio A. Carrasco*, Erika Meerhoff, Beatriz Yannicelly, and Christian M. Ibáñez Abstract New records of early-life stages of cephalopods are presented based on planktonic collections carried out around Easter Island (Rapa Nui; 27°7′S; 109°21′W) and at the nearby Apolo Seamount (located at ∼7 nautical miles southwest from Easter Island) during March and September 2015 and March 2016. A total of thirteen individuals were collected, comprising four families (Octopodidae, Ommastrephidae, Chtenopterygidae, and Enoploteuthidae) and five potential genera/types (Octopus sp., Chtenopteryx sp., rhynchoteuthion paralarvae, and two undetermined Enoploteuthid paralarvae). Cephalopod mantle lengths (ML) ranged from 0.8 to 4.5 mm, with 65% of them (mainly Octopodidae) corresponding to newly hatched paralarvae of ~1 mm ML, and 35% to rhynchoteuthion and early stages of oceanic squids of around 1.5 - 4.5 mm ML. These results provide the first records on composition and presence of early stages of cephalopods around a remote Chilean Pacific Island, while also providing a morphological and molecular basis to validate the identity of Octopus rapanui (but not Callistoctopus, as currently recorded), Ommastrephes bartramii and Chtenopteryx sp. around Rapa Nui waters. Despite adult Octopodidae and Ommastrephidae have been previously recorded at these latitudes, the current findings provide evidence to suggest that the northwest side of Easter Island, and one of the nearby seamounts, may provide a suitable spawning ground for benthic and pelagic species of cephalopods inhabiting these areas. For Chtenopterygidae and Enoploteuthidae, this is the first record for the Rapa Nui ecoregion. -

Part I. Revision of Buttsoceras. Part II. Notes on the Michelinoceratida
MEMOIR 10 PART I Revision of Buttsoceras PART II Notes on the Michelinoceratida By ROUSSEAU H. FLOWER 1 9 6 2 STATE BUREAU OF MINES AND MINERAL RESOURCES NEW MEXICO INSTITUTE OF MINING AND TECHNOLOGY CAMPUS STATION SOCORRO, NEW MEXICO NEW MEXICO INSTITUTE OF MINING & TECHNOLOGY E. J. Workman, President STATE BUREAU OF MINES AND MINERAL RESOURCES Alvin J. Thompson, Director THE REGENTS MEMBERS Ex OFFICIO The Honorable Edwin L. Mechem ........................................ Governor of New Mexico Tom Wiley .......................................................... Superintendent of Public Instruction APPOINTED MEMBERS William G. Abbott ............................................................................................... Hobbs Holm 0. Bursum, Jr. ......................................................................................... Socorro Thomas M. Cramer ......................................................................................... Carlsbad Frank C. DiLuzio ...................................................................................... Albuquerque Eva M. Larrazolo (Mrs. Paul F.) ............................................................... Albuquerque Published October I2, 1962 For Sale by the New Mexico Bureau of Mines & Mineral Resources Campus Station, Socorro, N. Mex.—Price $2.00 Contents PART I REVISION OF BUTTSOCERAS Page ABSTRACT ....................................................................................................................... INTRODUCTION ............................................................................................................................. -

Peter D. Ward – Curriculum Vitae Born May 12, 1949, Seattle Washington, USA Married, Two Children
1 Peter D. Ward – Curriculum Vitae Born May 12, 1949, Seattle Washington, USA Married, two children 1. Education B.S., 1971 Interdisciplinary Studies (Paleoecology), University of Washington, Seattle M.S., 1973 Geology, University of Washington, Seattle Ph.D., 1976 Geology, McMaster University, Hamilton, Ontario, Canada Employment 1976-1978 Assistant Professor, Department of Geology, Ohio State University 1978-1981 Assistant Professor, Department of Geology, University of California, Davis 1981-1983 Associate Professor with Tenure, Department of Geology and Division of Environmental Sciences, University of California, Davis 1982 Visiting Scientist, Laboratoire Arago (C.N.R.S.), Banyuls, France 1984 Professor, Department of Geology, University of California, Davis 1985 Associate Professor, University of Washington 1986 Professor, Department of Geological Sciences, and Adjunct Professor, Department of Zoology, University of Washington 1989- 1996 Curator of Invertebrate Paleontology, Thomas Burke Memorial Museum, University of Washington 1991. Visiting Scientist, South African Museum 1992-1996 Chairman and Head, Division of Geology and Paleontology, Thomas Burke Memorial Museum, University of Washington 2000. Visiting Scientist, South African Museum 2001 – present, Adjunct Professor, Department of Astronomy, University of Washington 2003- present, Professor, Dept. of Biology, and Professor, Department of Earth and Space Sciences, The University of Washington 2007- Adjunct Curator, Burke Museum of Natural History and Culture, University of -

OREGON ESTUARINE INVERTEBRATES an Illustrated Guide to the Common and Important Invertebrate Animals
OREGON ESTUARINE INVERTEBRATES An Illustrated Guide to the Common and Important Invertebrate Animals By Paul Rudy, Jr. Lynn Hay Rudy Oregon Institute of Marine Biology University of Oregon Charleston, Oregon 97420 Contract No. 79-111 Project Officer Jay F. Watson U.S. Fish and Wildlife Service 500 N.E. Multnomah Street Portland, Oregon 97232 Performed for National Coastal Ecosystems Team Office of Biological Services Fish and Wildlife Service U.S. Department of Interior Washington, D.C. 20240 Table of Contents Introduction CNIDARIA Hydrozoa Aequorea aequorea ................................................................ 6 Obelia longissima .................................................................. 8 Polyorchis penicillatus 10 Tubularia crocea ................................................................. 12 Anthozoa Anthopleura artemisia ................................. 14 Anthopleura elegantissima .................................................. 16 Haliplanella luciae .................................................................. 18 Nematostella vectensis ......................................................... 20 Metridium senile .................................................................... 22 NEMERTEA Amphiporus imparispinosus ................................................ 24 Carinoma mutabilis ................................................................ 26 Cerebratulus californiensis .................................................. 28 Lineus ruber ......................................................................... -

Version of the Manuscript
Accepted Manuscript Antarctic and sub-Antarctic Nacella limpets reveal novel evolutionary charac- teristics of mitochondrial genomes in Patellogastropoda Juan D. Gaitán-Espitia, Claudio A. González-Wevar, Elie Poulin, Leyla Cardenas PII: S1055-7903(17)30583-3 DOI: https://doi.org/10.1016/j.ympev.2018.10.036 Reference: YMPEV 6324 To appear in: Molecular Phylogenetics and Evolution Received Date: 15 August 2017 Revised Date: 23 July 2018 Accepted Date: 30 October 2018 Please cite this article as: Gaitán-Espitia, J.D., González-Wevar, C.A., Poulin, E., Cardenas, L., Antarctic and sub- Antarctic Nacella limpets reveal novel evolutionary characteristics of mitochondrial genomes in Patellogastropoda, Molecular Phylogenetics and Evolution (2018), doi: https://doi.org/10.1016/j.ympev.2018.10.036 This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain. Version: 23-07-2018 SHORT COMMUNICATION Running head: mitogenomes Nacella limpets Antarctic and sub-Antarctic Nacella limpets reveal novel evolutionary characteristics of mitochondrial genomes in Patellogastropoda Juan D. Gaitán-Espitia1,2,3*; Claudio A. González-Wevar4,5; Elie Poulin5 & Leyla Cardenas3 1 The Swire Institute of Marine Science and School of Biological Sciences, The University of Hong Kong, Pokfulam, Hong Kong, China 2 CSIRO Oceans and Atmosphere, GPO Box 1538, Hobart 7001, TAS, Australia. -

Octopus Insularis</Italic> As a New Marine Model for Evolutionary
© 2019. Published by The Company of Biologists Ltd | Biology Open (2019) 8, bio046086. doi:10.1242/bio.046086 RESEARCH ARTICLE Octopus insularis as a new marine model for evolutionary developmental biology Ernesto Maldonado1,*, Emma Rangel-Huerta1,2, Roberto González-Gómez3,4, Gabriel Fajardo-Alvarado3,4 and Piedad S. Morillo-Velarde4,5,* ABSTRACT of aquatic animal eggs and embryos guarantees the observation of Octopuses are intriguing organisms that, together with squids and every developmental stage using microscopy and allows detailed cuttlefishes, form the extant coleoid cephalopods. This group includes experimental analysis from the first cell division through to the many species that can potentially be used as models in the fields of formation of embryonic germ layers and organogenesis (Boletzky biomedicine, developmental biology, evolution, neuroscience and et al., 2006). Finally, small embryos allow reasonable sample sizes even for robotics research. The purpose of this work is to first to be tested together using multi-well plates to provide multiple present a simple method for maintaining Octopus insularis embryos experimental replicates at the same time, making them cost- under a laboratory setup. Second, we show that these embryos are effective animal models (Hill et al., 2005). suitable for detailed analyses of specific traits that appear during Coleoid cephalopods (octopus, squid and cuttlefish) exhibit the developmental stages, including the eyes, hearts, arms, suckers, largest nervous systems found among invertebrates (Young, 1971) chromatophores and Kölliker’s organs. Similar complex traits between and a sophisticated visual system controlling body color changes for cephalopods and vertebrates such as the visual, cardiovascular, communication, camouflage and mimicry (Hanlon et al., 2011; neural and pigmentation systems are generally considered to be a Robin et al., 2014). -

Abstract Volume
ABSTRACT VOLUME August 11-16, 2019 1 2 Table of Contents Pages Acknowledgements……………………………………………………………………………………………...1 Abstracts Symposia and Contributed talks……………………….……………………………………………3-225 Poster Presentations…………………………………………………………………………………226-291 3 Venom Evolution of West African Cone Snails (Gastropoda: Conidae) Samuel Abalde*1, Manuel J. Tenorio2, Carlos M. L. Afonso3, and Rafael Zardoya1 1Museo Nacional de Ciencias Naturales (MNCN-CSIC), Departamento de Biodiversidad y Biologia Evolutiva 2Universidad de Cadiz, Departamento CMIM y Química Inorgánica – Instituto de Biomoléculas (INBIO) 3Universidade do Algarve, Centre of Marine Sciences (CCMAR) Cone snails form one of the most diverse families of marine animals, including more than 900 species classified into almost ninety different (sub)genera. Conids are well known for being active predators on worms, fishes, and even other snails. Cones are venomous gastropods, meaning that they use a sophisticated cocktail of hundreds of toxins, named conotoxins, to subdue their prey. Although this venom has been studied for decades, most of the effort has been focused on Indo-Pacific species. Thus far, Atlantic species have received little attention despite recent radiations have led to a hotspot of diversity in West Africa, with high levels of endemic species. In fact, the Atlantic Chelyconus ermineus is thought to represent an adaptation to piscivory independent from the Indo-Pacific species and is, therefore, key to understanding the basis of this diet specialization. We studied the transcriptomes of the venom gland of three individuals of C. ermineus. The venom repertoire of this species included more than 300 conotoxin precursors, which could be ascribed to 33 known and 22 new (unassigned) protein superfamilies, respectively. Most abundant superfamilies were T, W, O1, M, O2, and Z, accounting for 57% of all detected diversity. -

Spineless Spineless Rachael Kemp and Jonathan E
Spineless Status and trends of the world’s invertebrates Edited by Ben Collen, Monika Böhm, Rachael Kemp and Jonathan E. M. Baillie Spineless Spineless Status and trends of the world’s invertebrates of the world’s Status and trends Spineless Status and trends of the world’s invertebrates Edited by Ben Collen, Monika Böhm, Rachael Kemp and Jonathan E. M. Baillie Disclaimer The designation of the geographic entities in this report, and the presentation of the material, do not imply the expressions of any opinion on the part of ZSL, IUCN or Wildscreen concerning the legal status of any country, territory, area, or its authorities, or concerning the delimitation of its frontiers or boundaries. Citation Collen B, Böhm M, Kemp R & Baillie JEM (2012) Spineless: status and trends of the world’s invertebrates. Zoological Society of London, United Kingdom ISBN 978-0-900881-68-8 Spineless: status and trends of the world’s invertebrates (paperback) 978-0-900881-70-1 Spineless: status and trends of the world’s invertebrates (online version) Editors Ben Collen, Monika Böhm, Rachael Kemp and Jonathan E. M. Baillie Zoological Society of London Founded in 1826, the Zoological Society of London (ZSL) is an international scientifi c, conservation and educational charity: our key role is the conservation of animals and their habitats. www.zsl.org International Union for Conservation of Nature International Union for Conservation of Nature (IUCN) helps the world fi nd pragmatic solutions to our most pressing environment and development challenges. www.iucn.org Wildscreen Wildscreen is a UK-based charity, whose mission is to use the power of wildlife imagery to inspire the global community to discover, value and protect the natural world.