Modern Drying Technology, Process Intensification ( Pdfdrive.Com ).Pdf
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Ocean Noise Strategy Roadmap
Ocean Noise Strategy Roadmap Ocean Noise Strategy Roadmap Jason Gedamke, Jolie Harrison, Leila Hatch, Robyn Angliss, Jay Barlow, Catherine Berchok, Chris Caldow, Manuel Castellote, Danielle Cholewiak, Monica L. DeAngelis, Robert Dziak, Ellen Garland, Shane Guan, Sean Hastings, Marla Holt, Ben Laws, David Mellinger, Sue Moore, Thomas J. Moore, Erin Oleson, Jacqueline Pearson-Meyer, Wendy Piniak, Jessica Redfern, Teri Rowles, Amy Scholik-Schlomer, Aileen Smith, Melissa Soldevilla, John Stadler, Sofie Van Parijs, Charles Wahle ii Table of Contents Executive Summary 1 Chapter 1—The NOAA Ocean Noise Strategy and Managed Species 6 Chapter 2—Can You Hear Me Here? Managing Acoustic Habitat in U.S. Waters 27 Chapter 3—Enhancing NOAA’s Ability to Characterize Aquatic Soundscapes 45 Chapter 4— NOAA Ocean Noise Strategy Implementation Case Studies 60 Case Study 1: Assessing the Risk of Chronic Noise from Commercial Ships to 63 Large Whale Acoustic Habitat Case Study 2: Managing Noise Impacts on Spawning Areas Used by 81 Acoustically Sensitive and Commercially Important Fish and Invertebrate Species Appendix A— The Status of Science for Assessing Noise Impacts on NOAA-Managed 91 Species Appendix B— Presence, Abundance, Distribution, Density, Habitat Use and 113 Population Trends Appendix C— Spreadsheet of Potential Authorities (e.g. Statutes, Executive Orders) 121 to Address Ocean Noise Issues Appendix D— NOAA Ocean Noise Strategy Cross-Office Team 138 iii List of Figures Page Figure 1-1. Potential Consequences of Disturbance conceptual model of the relationships 11 linking disturbance to changes in behavior and physiology, health, vital rates, and population dynamics. Figure 2-1. Illustration of potential acoustically-mediated information in a marine 28 community. -

Telstar Satellite Rocket Launched
Today ' 3 i' ^^^^ ^ 21,950 lafad. Lej» tod|bt In the 4k, 9M' Weratr, page 2.' DIAL SH 1-0010 VOL. '85 NO 224. IMIM* itltr. MoniStT thronm frliUr. B^oond oitu Pwttf* rvu °3' «'-'• •"* raid at KM Buk and at ASditiooU XUllnc OtOei. RED BANK, N. J., TUESDAY, MAY 7, 1963 7c PER COPY PAGE ONE Senate to Probe Race) Tracks' Expenses TRENTON (AP)-The ttate sen- Hillery, R-Merris and Wayne Du- bill and that was why he support- the committee membership and Sandman on what basis the com- ate has deckled to do tome of moot, It-Warren, sponsored a ed Stamler's. < warned jokingly that any commit- mittee members were chosen. tee member caught with white- its own Investigating into a $2.4 measure calling for a similar In- "What he meant by not enough He said no committee member million expense account submit- vestigation, but their resolution votes for my bill is that he wash on his fingers would be bounced from the committee. could be running for re-election ted by two race tracks lor operat- did not include Cowgili and was couldn't get six votes in the GOP and have a race track in his caucus.. .there were 10 Demo- He-named Dumont as chairman ing a special 30-day season last limited to a senate committee of county. year to provide money for shore five, crats ready to vote for it here," and appointed Sens. Hillery; Stam- relief. Gives Reason Cowgili said. ler; waiiam F. Kelly, D-Hud- The only senator who,is run- The senate, after a heated ex- Sandman said on the senate floor As soon as the vote was tak- son, and John A. -

Porównanie Skuteczności Urządzeń Oxybaria S I Laserobaria S W Skojarzonym Leczeniu Fizykalnym Owrzodzeń Kończyn Dolnych
Lekarz Mikołaj Pietrzak Porównanie skuteczności urządzeń Oxybaria S i Laserobaria S w skojarzonym leczeniu fizykalnym owrzodzeń kończyn dolnych Rozprawa na stopień doktora nauk medycznych Promotor: prof. dr hab. n. med. Grzegorz Cieślar Katedra i Oddział Kliniczny Chorób Wewnętrznych, Angiologii i Medycyny Fizykalnej Kierownik Katedry: prof. dr hab. n. med. Grzegorz Cieślar Wydział Lekarski z Oddziałem Lekarsko-Dentystycznym w Zabrzu Śląski Uniwersytet Medyczny w Katowicach Zabrze 2019 Pobrano z Repozytorium Śląskiego Uniwersytetu Medycznego w Katowicach / Downloaded from Repository of Medical University of Silesia 2021-09-26 Mikołaj Pietrzak Oddział Kliniczny Chorób Wewnętrznych, Angiologii i Medycyny Fizykalnej Szpitala Specjalistycznego nr 2 w Bytomiu 41-902 Bytom, ul. Batorego 15 e-mail: [email protected] RECENZENCI: Prof. dr hab. n. med. Andrzej Szuba Katedra i Klinika Angiologii, Nadciśnienia Tętniczego i Diabetologii Wydział Lekarski Kształcenia Podyplomowego Uniwersytet Medyczny im. Piastów Śląskich we Wrocławiu Dr hab. n. o kult. fiz. Jan Szczegielniak Prof. nadzw. PO Katedra Fizjoterapii Klinicznej Instytut Fizjoterapii Wydział Wychowania Fizycznego i Fizjoterapii Politechnika Opolska w Opolu 1 Pobrano z Repozytorium Śląskiego Uniwersytetu Medycznego w Katowicach / Downloaded from Repository of Medical University of Silesia 2021-09-26 SPIS TREŚCI WYKAZ STOSOWANYCH SKRÓTÓW…………………………………………. 4 WYKAZ TABEL……………………………………………………………………. 6 WYKAZ RYCIN…………………………………………………………………….. 8 1. WSTĘP…………………………………………………………………………. 9 1.1. Rany przewlekłe………………………………………………………….... 9 1.1.1. Definicja, epidemiologia, powikłania……………………………….. 9 1.1.2. Etiopatogeneza owrzodzeń w obrębie kończyn dolnych ………… 10 1.1.3. Proces gojenia ran przewlekłych…………………………………… 13 1.1.4. Leczenie owrzodzeń w obrębie kończyn dolnych ………………… 15 1.1.5. Rokowanie w przypadku owrzodzeń w obrębie kończyn dolnych 16 1.2. Wybrane metody fizykalne stosowane w leczeniu owrzodzeń kończyn dolnych……………………………………………………………………… 17 1.3. -
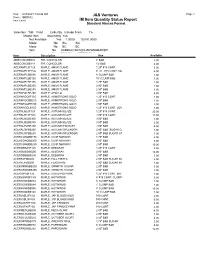
J&S Ventures IM Item Quantity Status Report
Run : 9/27/2021 7:20:46 AM J&S Ventures Page 1 Form : IMR0032 Item Level IM Item Quantity Status Report Standard Abecas Format Selection : Tab Field Order By Include From To Master Item Ascending Yes Net Available Yes 1.0000 10,000.0000 Mode No BG BG Mode No BC BC Item No HARBALCAR100 HARWEEBARSPI Item No ZZAVAIL ZZTRESTAK Item Description Size Available ABIECONCBB072 FIR, CONCOLOR 6' B&B 2.00 ABIECONCBB144 FIR, CONCOLOR 12' B&B 2.00 ACERAMFL0F125 MAPLE, AMUR FLAME 1.25" #15 CONT. 5.00 ACERAMFL0F15G MAPLE, AMUR FLAME 6' CL. #15 CONT. MULTI STEM 4.00 ACERAMFLBB096 MAPLE, AMUR FLAME 8' CLUMP B&B 1.00 ACERAMFLBB120 MAPLE, AMUR FLAME 10' CLUMP B&B 1.00 ACERAMFLBB175 MAPLE, AMUR FLAME 1.75" B&B 1.00 ACERAMFLBB200 MAPLE, AMUR FLAME 2.00" B&B 1.00 ACERAMFLBB250 MAPLE, AMUR FLAME 2.50" B&B 1.00 ACERAPOLBB200 MAPLE, APOLLO 2.00" B&B 5.00 ACERARGO0F125 MAPLE, ARMSTRONG GOLD 1.25" #15 CONT. 1.00 ACERARGOBB200 MAPLE, ARMSTRONG GOLD 2.00" B&B 1.00 ACERARGOBB250 MAPLE, ARMSTRONG GOLD 2.50" B&B 2.00 ACERARGOLB125 MAPLE, ARMSTRONG GOLD 1.25" #15 CONT. LOW BRANCH 1.00 ACERAUBL0F125 MAPLE, AUTUMN BLAZE 1.25" #15 CONT. 20.00 ACERAUBL0F20G MAPLE, AUTUMN BLAZE 2.00" #20 CONT. 17.00 ACERAUBLBB300 MAPLE, AUTUMN BLAZE 3.00" B&B 1.00 ACERAUBLBB350 MAPLE, AUTUMN BLAZE 3.50" B&B 2.00 ACERAUFABB200 MAPLE, AUTUMN FANTASY 2.00" B&B 4.00 ACERAUSPBB200 MAPLE, AUTUMN SPLENDOR 2.00" B&B SUGAR CADDO 1.00 ACERAUSPBB250 MAPLE, AUTUMN SPLENDOR 2.50" B&B SUGAR CADDO 1.00 ACERCONOBB175 MAPLE, CLNR NORWAY 1.75" B&B 2.00 ACERCONOBB200 MAPLE, CLNR NORWAY 2.00" B&B 5.00 ACERCONOBB250 MAPLE, CLNR NORWAY 2.50" B&B 19.00 ACERDEBO0F125 MAPLE, DEBORAH 1.25" #15 CONT. -

Nonlinear Ultrasound Spectroscopy for Chemical Quantification
Nonlinear Ultrasound Spectroscopy for Chemical Quantification Jonathan R. Dion Department of Chemistry McGill University, Montreal Quebec, Canada August 2011 A thesis submitted to McGill University in partial fulfillment of the requirements of the degree of Doctor of Philosophy © Jonathan R. Dion, 2011 Abstract Analyte quantification is an area of great practical interest for biomedical and commercial applications. Measurement in highly scattering media such as tissue, blood, and other biological fluids is challenging using conventional spectroscopic methods. Likewise, measurement of compounds on-line or in opaque containers can be extremely challenging. This thesis presents general approaches for analyte quantification in liquids by the frequency analysis of ultrasound waves. Initially, this research focused on the quantification of analytes using dispersive hydrogel sensors. These sub-micron hydrogels vibrate at characteristic resonance frequencies when exposed to ultrasound. The resonance frequencies of the sensors could be modulated by generating molecularly imprinted pockets that would recognize and bind to molecules. Changes in the measured ultrasound frequency spectrum upon binding could then be detected. Using this approach, quantification of theophylline between 10 μM and 6.1 mM was shown. Improvements in analyte quantification using ultrasound were made by designing hydrogel sensors coupled to antibodies. The high affinity of antibodies for a specific antigen allowed the quantification of acetaminophen between 3.5 nM and 20.8 nM. The highly selective antibodies were also demonstrated to allow acetaminophen determination in a variety of biological media. Though the ultrasound frequency profiles change in the different media, frequencies used for multilinear determination of the analyte were ii clustered in certain regions of the spectrum. -

Audio and Tactile Interfaces to Mobility Aids for the Blind
Interfaces for multi-sensor systems for navigation for the blind Penny Probert, David Lee and Gordon Kao Robotics Research Group, Department of Engineering Science, University of Oxford, 19 Parks Road, Oxford, OX1 3PJ, ENGLAND Tel: +44-1865-273926 Fax: +44-1865-273908 [email protected], [email protected] ABSTRACT This paper describes work towards a multi-sensor system to assist blind people to move around an urban or indoor environment. The communication of navigation information is constrained by both the type of information imparted and, in many ways more crucially, by the type of information which real sensors can extract. In this paper we describe the use of ultrasound to provide two types of information: first the low level directional information which may be provided currently by a guide dog and then information on features to allow the blind person to locate and direct themselves within the environment. A directional system with a simple vibrator interface is described and its performance assessed by a group of users. Finally we discuss the possibility of feature extraction for navigation by recognition and give some results using frequency modulated sonar. Keywords: ultrasound, mobility aids, blindness, human interface 1. BACKGROUND AND STRUCTURE OF PAPER Vision is by far the most important sense for most people, and trends in virtual reality and computer interfaces reflect this emphasis. Sighted people appreciate detailed images and are able to abstract relevant data from images with ease, and the challenge in virtual reality is not in abstracting the data but providing enough detail at sufficient speed. -

Kibo HANDBOOK
Kibo HANDBOOK September 2007 Japan Aerospace Exploration Agency (JAXA) Human Space Systems and Utilization Program Group Kibo HANDBOOK Contents 1. Background on Development of Kibo ............................................1-1 1.1 Summary ........................................................................................................................... 1-2 1.2 International Space Station (ISS) Program ........................................................................ 1-2 1.2.1 Outline.........................................................................................................................1-2 1.3 Background of Kibo Development...................................................................................... 1-4 2. Kibo Elements...................................................................................2-1 2.1 Kibo Elements.................................................................................................................... 2-2 2.1.1 Pressurized Module (PM)............................................................................................ 2-3 2.1.2 Experiment Logistics Module - Pressurized Section (ELM-PS)................................... 2-4 2.1.3 Exposed Facility (EF) .................................................................................................. 2-5 2.1.4 Experiment Logistics Module - Exposed Section (ELM-ES)........................................ 2-6 2.1.5 JEM Remote Manipulator System (JEMRMS)............................................................ -

Theory and Simulation of Streaming and Radiation Forces at Ultrasound Resonances in Microfluidic Devices
Downloaded from orbit.dtu.dk on: Dec 17, 2017 Acoustofluidics: Theory and simulation of streaming and radiation forces at ultrasound resonances in microfluidic devices Bruus, Henrik Published in: Acoustical Society of America. Journal Publication date: 2009 Document Version Publisher's PDF, also known as Version of record Link back to DTU Orbit Citation (APA): Bruus, H. (2009). Acoustofluidics: Theory and simulation of streaming and radiation forces at ultrasound resonances in microfluidic devices. Acoustical Society of America. Journal, 125(4), 2592-2592. General rights Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. • Users may download and print one copy of any publication from the public portal for the purpose of private study or research. • You may not further distribute the material or use it for any profit-making activity or commercial gain • You may freely distribute the URL identifying the publication in the public portal If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. TUESDAY MORNING, 19 MAY 2009 GRAND BALLROOM II, 8:00 A.M. TO 12:00 NOON Session 2aAAa Architectural Acoustics: Multiple Channel Systems in Room Acoustics Ning Xiang, Cochair Architecture, Rensselaer Polytechnic Inst., Troy, NY 12180 Boaz Rafaely, Cochair Electrical and Computer Engineering Dept., Ben Gurion Univ., Beer Sheva, Israel Chair’s Introduction—8:00 Invited Papers 2a TUE. -

Physiology of Decompressive Stress
CHAPTER 3 Physiology of Decompressive Stress Jan Stepanek and James T. Webb ... upon the withdrawing of air ...the little bubbles generated upon the absence of air in the blood juices, and soft parts of the body, may by their vast numbers, and their conspiring distension, variously streighten in some places and stretch in others, the vessels, especially the smaller ones, that convey the blood and nourishment: and so by choaking up some passages, ... disturb or hinder the circulation of the blouod? Not to mention the pains that such distensions may cause in some nerves and membranous parts.. —Sir Robert Boyle, 1670, Philosophical transactions Since Robert Boyle made his astute observations in the Chapter 2, for details on the operational space environment 17th century, humans have ventured into the highest levels and the potential problems with decompressive stress see of the atmosphere and beyond and have encountered Chapter 10, and for diving related problems the reader problems that have their basis in the physics that govern this is encouraged to consult diving and hyperbaric medicine environment, in particular the gas laws. The main problems monographs. that humans face when going at altitude are changes in the gas volume within body cavities (Boyle’s law) with changes in ambient pressure, as well as clinical phenomena THE ATMOSPHERE secondary to formation of bubbles in body tissues (Henry’s law) secondary to significant decreases in ambient pressure. Introduction In the operational aerospace setting, these circumstances are Variations in Earthbound environmental conditions place of concern in high-altitude flight (nonpressurized aircraft limits and requirements on our activities. -

2011 Annual Report
Rochester Center for Biomedical Ultrasound icrobu ng m bble c eeri ont gin ras en t a e ge su n is ts t t E s standin i ect g w ss IP ef av u io e f e W b e c S ld h s U a y F r h I S a ling wa M c p H ve w t a a s r o U e r c n g r g o R i n g e z o i n l F t a a g i s s t g a t i a a o A o l e m g n i m R i r a F y c n s p c i I c t o h n i s n y e n o i l n u o n i t n q t g h r e a m e e i a r r a r f d v a a - r p a h h e s d a u n c g b t c i u i c o u s u a l r t o u h l s u a r s u t g n i l o t c n i r i t s a c r s e o r e u r D t o n t c d n o a o a i t b a e r r c p s p c l i e t r s u a o c c o a u s n t i o c i t r a a t d i v i a a t c i o n e c f r o 2011Annual Report RCBU laboratories are advancing biomedical ultrasound across many topics in diagnostic imaging, ultrasound therapy, and fundamental acoustics. -

The Imaging Sciences Ultrasound Department Experienced More Than
This year’s annual report The Imaging Sciences summarizes progress from Ultrasound Department RCBU laboratories across experienced more than 5% diverse topics in biomedical growth in exam and patient ultrasound imaging and volumes in 2009; performing therapy. The cover and related over 20,000 exams. The stories inside this report unit continued to expand describe advances by RCBU its clinical coverage; adding members on novel applications more sonographer positions to of ultrasound for tissue manage the increased demand. engineering and regenerative In the spring of 2009, medicine. The RCBU continues GE’s newest platform, the Logic to advance the development of E9, was installed; replacing elastography techniques. Included within this report the existing Logic9 units. The new machines provide are highlights of innovations in sonoelastography and enhanced image quality and improved workflow; new techniques based on acoustic radiation force. This allowing sonographers to merge real-time ultrasound with year also marked the naming of the UR Hajim School of previously acquired CT, MR, or ultrasound images. Engineering and Applied Sciences. The University of Rochester Medical Center was The RCBU continues to play a prominent role represented by sonographers and physicians in education in clinical and technological advances in the use of nationally and internationally. As faculty for Armed ultrasound for diagnostic imaging and therapy. Nonlinear Forces Institute of Pathology, Washington DC, Dr. imaging techniques, sonoelastography, and ultrasound Rubens continued to teach courses on spleen, testis, contrast agents all have foundations from innovations scrotum, portal Doppler, and testicular Doppler. Drs. within RCBU laboratories. Highlights of the Eighth Bhatt, Dogra, Rubens, Strang, and Voci also participated International Conference on Ultrasonic Measurement as faculty at the Radiological Society of North America and Imaging of Tissue Elasticity are reviewed in this (RSNA), the American Institute of Ultrasound in Medicine report. -

Modificaciones En Factores Relacionados Con El Aroma Y La Textura De La Manzana, Melocotón Y Nectarina Durante La Maduración Y La Post- Cosecha
Modificaciones en factores relacionados con el aroma y la textura de la manzana, melocotón y nectarina durante la maduración y la post- cosecha Abel Ortiz Catalán R " Q $% R &' ' ()$ + , -.)/ " 1 2 - 234 $61Q&' ' ( )$ + ,R$ 6 $ Q $ % R 1 $ 2 ( , ) 1 + -7 8 9 8 92 - ) , ' 2 -.)/ 2 - 234 $&:;<< " " " # " "%& & " ' " % " % % ( '" * % & ! # # ! ! $ + , % & % & '! $ ! ( # $ ! $ & " ( &" -./ ) ' * !! 9 ! $Q, - R / 0, 12 3 4 5 $6/-73%(8 ' & ! 9) $Q:!;<=!>R / 0, 12 3 *1 9)9 ' $ ! ! %) ! $Q-2 R / 04 ,2 2, 12 3 ' ' / ! $ Q:!; ) <=!>R / 04 ,, 12 3 *112 9))> ' / 7 $ $ ! #! # >) Q, - R $ # ! / 04 ,, 12 3 *11 9 ' / / '! $Q- 2 R $ ! $ 0 ( 7 $ / 04 ,, 12 3 *112 9(9) ' / ? ! $ # $ ! ! # Q- 2 R / 04 ,, 12 22 3 ' ' / * Q7 # R $ ! & / 0, 12 24 ,2 3 *11 %)>( ' 3 @ $ ! ! $Q, - R / 04 ,, 12 3 *11 >((% ' 3 & ! # @ $ @ # $Q:!;<=!>R / 0, 12 3 *1 A ((%B; #((( ' ' 3 @ #! $ $ % Q?